What is ClinicalTrials.gov?
ClinicalTrials.gov is a resource that provides access to information on clinical trials studying a wide range of diseases, conditions and interventions. Studies listed in the database are conducted in all 50 States and worldwide. Each ClinicalTrials.gov record includes summary information about studies.
Want a quick start guide to CT.gov?
Problems with CT.gov Record?
Registration Requirements
ClinicalTrials.gov Registration Decision Tool
Use the decision tool to determine if registration on CT.gov is required
Registration may be required if one (or more) of the following is true:
NIH funded clinical trials are required to be registered:
Per NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information - Effective January 18, 2017, if your study is NIH funded and meets the NIH definition of a clinical trial, then clinicaltrials.gov registration is required.
- For NIH funded research, use the following four questions to determine the difference between a clinical study and a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to one or more interventions?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
- If the answers to ALL 4 questions are “yes”, your study meets the NIH definition of a clinical trial.
- Clinical trials subject to the regulation are generally called "applicable clinical trials."
- Applicable clinical trials are required to be registered in ClinicalTrials.gov not later than 21 calendar days after the enrollment of the first participant.
Resources:
- Decision Tree: to help determine if you NIH funded study requires clinicaltrials.gov registration
- Podcast: What NIH Researchers and Recipients Should Know about ClinicalTrials.gov
NIH Definition of an “INTERVENTION”:
An "intervention" is defined as a manipulation of the subject or subject’s environment for the purpose of modifying one or more health-related biomedical or behavioral processes and/or endpoints.
Examples include:
- drugs/small molecules/compounds;
- biologics; devices;
- procedures (e.g., surgical techniques);
- delivery systems (e.g., telemedicine, face-to-face interviews);
- strategies to change health-related behavior (e.g., diet, cognitive therapy, exercise training, development of new habits);
- treatment strategies; prevention strategies; and diagnostic strategies.
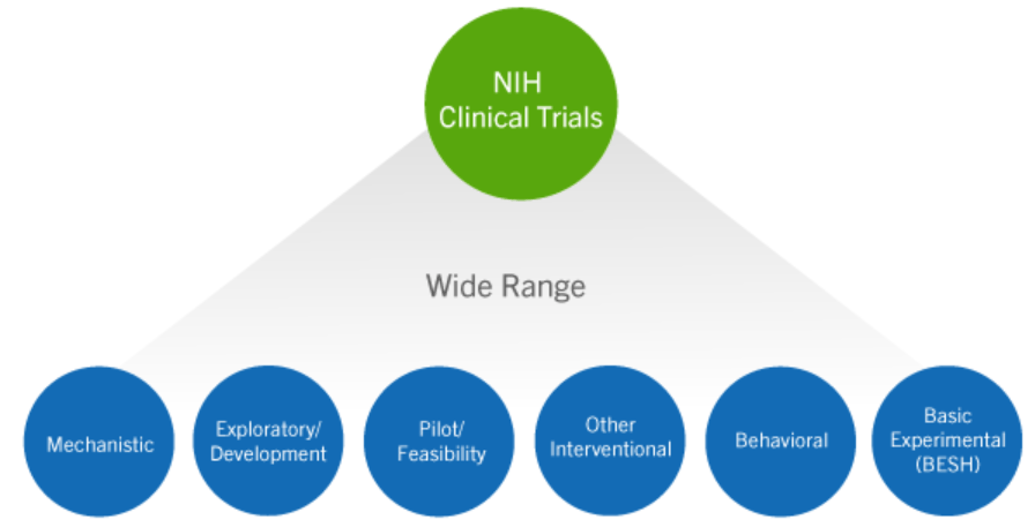
This Includes
- studies with healthy participants
– Phase 1 trials of FDA-regulated drugs and biological products
– Small feasibility studies of FDA regulated device products
– studies with no comparison group (placebo or control)
– studies designed to assess the pharmacokinetics / safety of an investigational drug
– Studies where only one aim or sub-aim meets the clinical trial definition.
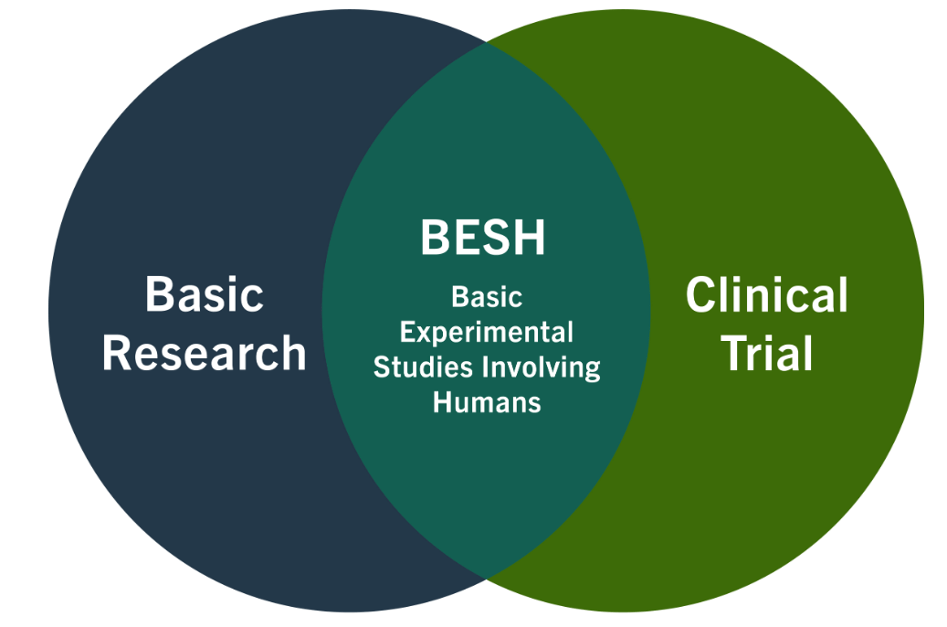
Basic experimental studies involving humans:
These meet both the definition of basic research and the NIH definition of a clinical trial.
- NIH published clinical trial case studies with examples of BESH (example - cases 16, 21, 23 and 31b).
- Example: healthy volunteers randomized to different durations of sleep deprivation where the dependent variable is stress hormone levels.
NIH Policy on Dissemination of NIH-Funded Clinical Trial Information:
- This policy applies to all NIH-funded clinical trials regardless of study phase, type of intervention, or whether they are subject to the regulation.
- As such, the policy encompasses all NIH-funded clinical trials, including applicable clinical trials subject to the regulation. All NIH-funded clinical trials will be expected to register and submit results information to ClinicalTrials.gov.
- In the regulation, results information includes participant flow, demographic and baseline characteristics, outcomes and statistical analyses, adverse events, the protocol and statistical analysis plan, and administrative information.
- In addition, informed consent documents for clinical trials are to include a specific statement relating to posting of clinical trial information at ClinicalTrials.gov.
Clinical Trial Indicator:
- The NIH Award Notice includes a "Clinical Trial Indicator" in Section IV which specifies whether the award supports any NIH defined clinical trials.
- If the Clinical Trial Indicator specifies "No" then your study does NOT meet the NIH definition of a clinical trial and therefore does not need to be registered on Clincaltrials.gov.
- If the Clinical Trial Indicator specifies "Yes" then your study meets the NIH definition of a clinical trial and must be registered on Clincaltrials.gov.
The Food and Drug Administration Amendments Act of 2007 (FDAAA) established legal requirements for sponsors and designated principal investigators (i.e., responsible parties) to report specified clinical trial information for certain ACT’s to ClinicalTrials.gov.
The requirements are designed to
- provide potential participants with information about trials of interest,
- reduce publication bias,
- help institutional review boards (IRBs) determine the appropriateness of a research study,
- and promote more efficient allocation of research funds.
**NEW RESOURCE: FDA slides of ClinicalTrials.gov Policies and Enforcement

Registration is also required if your study meets the DHHS definition of an Applicable Clinical Trial (ACT) These include:
- Controlled clinical investigations (other than phase 1 investigations) of any U.S. Food and Drug Administration (FDA) -regulated drug or biological product for any disease or condition
- It also includes certain studies of FDA-regulated medical devices, and FDA-required pediatric post market surveillances of a device product
Researchers can use the following tools to determine if their trial meets the DHHS definition of an ACT:
ACT – important definitions:
Is the study interventional (a clinical trial)?
- Participants are prospectively assigned to an intervention(s) to evaluate the effect of the intervention(s) on biomedical or other health-related outcomes. [Source: 42 CFR 11.10(a); 81 FR 65140-41]
Does the study evaluate* at least one U.S. FDA-regulated drug, biological, or device product?
FDA-regulated Device Product means:
- A device product subject to (1) a finding of substantial equivalence under section 510(k) of the FD&C Act, (2) under section 515 - requiring a premarket approval application (PMA) for the device product, or (3) a marketing application for a Humanitarian Use Device (HUD) - a Humanitarian Device Exemption under section 520(m) of the FD&C Act.
- Device products that are considered to be subject to section 510(k), 515, or 520(m) of the FD&C Act include significant risk devices (SR) for which approval of an IDE is required, non-significant risk devices (NSR) that subject to abbreviated IDE requirements (21 CFR 812.2(b)), or device products that are exempt from the submission requirements (IDE Exempt) of 21 CFR part 812.
- It DOES NOT include device feasibility, Phase 0 or Phase 1 trials.
*The device is under clinical investigation and is the object of the investigation.
FDA-regulated Drug Product means:
- A drug that is the subject of an approved NDA (new drug application) or BLA (biologic license application) or that would require an approved NDA or BLA to be legally marketed in the United States.
- A clinical trial including an intervention with a “dietary supplement” could be an ACT.
- It DOES NOT include Phase 0 or Phase 1 drug / biologic trials. For determining whether a drug / biological is the variable of interest, see page 8 of the ACT checklist
Sponsor-Investigators submitting an IND, NDA, BLA, or 510k etc. application to the FDA:
- The FDA recommends that Form FDA 3674 accompany the submission.
- Per instructions on Form FDA 3674, Sponsors must "provide the NCT Number obtained from www.ClinicalTrials.gov for each applicable clinical trial for which the sponsor/ applicant/submitter is the “responsible party” and for which data is included, relied upon, or otherwise referred to, in the application/submission which the certification accompanies."
- Box 10 may be left blank if the submitter has checked Box 9.C but, at the time the certification is completed, the submitter has not yet received any NCT number(s) for the applicable clinical trial(s) for which data is included, relied upon, or otherwise referred to in the IND application. There is no requirement to register the study on clinicaltirals.gov at this time!
- The only expectation for registration per FDA is outlined in 42 CFR 11.24(a):
- General. Except as provided in paragraph (b) of this section, the responsible party for an applicable clinical trial for which submission of clinical trial registration information is required must submit the clinical trial registration information specified in section 402(j)(2)(A)(ii) of the Public Health Service Act (42 U.S.C. 282(j)(2)(A)(ii)) or § 11.28(a), as applicable, not later than December 26, 2007, or 21 calendar days after the first human subject is enrolled, whichever date is later.
- Please note that an updated Form FDA 3674 providing the applicable clinical trial’s NCT number is not required to be submitted to the IND after the trial is registered on ClinicalTrials.gov. However, the trial’s NCT number may need to be included in a Form FDA 3674 that accompanies a future application or submission to FDA (e.g., the submission of a new protocol to the IND).
Registration Requirements per International Committee of Medical Journal Editors (ICMJE):

- In 2005, the ICMJE defined trials that must be registered in order to be considered for publication in journals that adhere to ICMJE standards.
- The ICMJE requires registration of clinical trials in a public trials registry at or before enrollment of the first participant to be considered for publication.
- Which journals are members of the ICMJE?
- Many journals (not limited to medical journals) have adopted the registration policy.** Editors requesting inclusion of their journal on the ICMJE website list of publications that follow ICMJE guidance listing implies enforcement by the journal of ICMJE’s trial registration policy.
The ICMJE definition of clinical trial: "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes.”
- With or without concurrent comparison or control groups,
- Health-related interventions are those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, and process-of-care changes.
- Health outcomes are any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events.
- …encourages registration of research with non-trial designs (e.g., observational studies)
- …journals will consider trials beginning on or after July 1, 2005 only if registration occurred before the first patient was enrolled ("prospective registration").
- …does not define the timing of first participant enrollment, but best practice dictates registration by the time of first participant consent.
- An acceptable registry must include the minimum 24-item trial registration data set at the time of registration.
**Those who are uncertain whether their trial meets the expanded ICMJE definition should err on the side of registration if they wish to seek publication in an ICMJE journal**
- Effective January 1, 2014, the Centers for Medicare and Medicaid Service (CMS) require the mandatory reporting of the ClinicalTrials.gov Identifier (NCT Number) number on claims for items and services provided in clinical trials that are qualified for coverage under the Medicare National Coverage Determination (NCD) Manual, Section 310.1
- Any claim that does not include the NCT number will be returned to the billing provider and may not be paid. If the study has been determined to be a Qualifying Clinical Trial (QCT) under Medicare’s NCD and the investigator intends to enroll Medicare beneficiaries, the study must be registered on ClinicalTrials.gov regardless if it meets the criteria for registration under the FDAAA, NIH and/or ICMJE requirements.

Department of Defense
Please check with your Program Officer if registration is required.

Patient-Centered Outcomes Research Institute (PCORI)
- Please review PCORI’s Process for Peer-Review of Primary Research and Public Release of Research Findings
- If your study qualifies as a clinical trial, registration is required prior to enrollment of the first patient.

NIH National Cancer Institute
- NEW RESOURCE: NCI Registration and Reporting Requirements Presentation
- All NIH-funded investigators including NCI investigators are subject to the NIH policy.
- “Covered Trials” means all initiated or commenced NCI-Supported Interventional Clinical Trials whether extramural or intramural.
- For every Covered Trial, Final Trial Results are expected to be reported in a publicly accessible manner within twelve (12) months of the Trial’s Primary Completion Date regardless of whether the clinical trial was completed as planned or terminated earlier.
To comply with the Policy, Final Trial Results may be reported in a publicly accessibly manner in various ways, which include but are not limited to ClinicalTrials.gov.

Department of Veterans Affairs
- Principal Investigators (PIs) of VHA Office of Research and Development (ORD) funded clinical trials are responsible for registering their trials with and submitting summary results to ClinicalTrials.gov, as a condition of funding.
- ORD uses the same definition of a clinical trial as the World Health Organization / ICMJE. This definition is "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes."
If you have a clinical trial that includes a drug that's available via expanded access:
Under FDA regulations (21 CFR 312.300), expanded access allows for the use of unapproved drugs and biologics outside of a clinical trial for patients with serious diseases or conditions when there is no satisfactory alternative therapy to treat the patient’s disease or condition. This is sometimes referred to as compassionate use or treatment use.
For an applicable drug clinical trial of an unapproved drug product the Public Health Service Act requires the submission of a separate expanded access record containing details about how to obtain access to the investigational product.
The final rule does not consider any expanded access use (i.e., access under treatment INDs or treatment protocols, which provide widespread access, access for intermediate-sized patient populations, or access for individual patients) to be an applicable clinical trial.
Note: Only a responsible party who is both the manufacturer of an investigational drug product or biological product and the sponsor of the trial can submit registration information for expanded access programs to ct.gov.
A responsible party who is not BOTH the manufacturer of the investigational drug product or biological product and the sponsor of the trial is not required to submit information for the Availability of Expanded Access data element (i.e., should select the response "unknown" for that data element). (81 FR 65052)
For example, a physician who submits an individual patient expanded access Investigational New Drug Application (IND), including for emergency use, as specified in 21 CFR 312.310, to the U.S. Food and Drug Administration generally would not be required to submit expanded access information to ClinicalTrials.gov.
How to Register & Update the Record
How to Register & Update the Record
The following user guide explains how to carry out some of the most common functions on ClinicalTrials.gov when registering a study. For a general overview of the registration process and requirements, see How to Register Your Study.
Protocol Registration and Results System (PRS) Guidance
EQUIP TIP: Submit to the IRB prior to releasing the clinicaltrials.gov record for PRS review
Once you have determined that your study needs to be registered on ClinicalTrials.gov, you will need to submit the record for the study in the Protocol Registration and Results System (PRS):
**PRS guided tutorials can be found here**
- For PRS access (if you do not already have an account), please contact Electronic Research Administration: era@research.uci.edu.
- If you are unsure if your study should be registered, contact your PRS Administrator:
- For UCI Health Science studies, please contact Jinah Chang (jinahec@hs.uci.edu )
- For UCI Cancer Center studies, please contact Michelle Tran (mdich@hs.uci.edu)
- For Non-UCI Health studies, please contact the EQUIP team (EQUIP@uci.edu).
- Once the account has been created, an email will be sent from ClinicalTrials.gov to the new user with a username, password and instructions for logging in to UCI’s Institutional PRS account
- For questions about the registration process at UCI, contact the EQUIP team (EQUIP@uci.edu), or contact PRS staff at register@clinicaltrials.gov.
- Go to https://register.clinicaltrials.gov/ to sign in
- Enter Organization: UCaliforniaIrvine
- Enter the username and password emailed to you by ClinicalTrials.gov

- Change your password once you log in for the first time
- Go to Accounts > Change password
- You will receive a temporary password via email
- Suggestion: Print or save a copy of the Protocol Data Entry Protocol Review Criteria (PDF) for reference. This document provides general guidance for compliant record creation as well as “Hints” detailing specific requirements for completion of key fields in the protocol record.
- Under “Quick Links”, select New Record or use the quick links to the left-hand side and click on “New Record”:

- Complete the “Create New Record” initiation screen
- Only one Record Owner can be assigned to a study record, but the Record Owner can allow other users to edit the study record by granting them access.
- For UCI Investigator Initiated studies, the Principal Investigator (Lead Researcher) or the responsible Clinical Research Coordinator can be listed as the “Record Owner”.
- Other study staff requiring PRS access should be listed in the “Access List”.

Click the Open button for the “Protocol Section"
**GENERAL EQUIP TIPS TO AVOID COMMON PRS ERRORS:
STUDY DESCRIPTION
- Brief Summary does not unnecessarily duplicate information provided for other data elements
- Brief Summary clearly states the study’s hypothesis or the purpose (for interventional and observational)
- Brief Summary and Detailed Description are written in complete sentences with no formatting errors
- Record does not use personal pronouns:“I, we, our, us, they, them, their” – becomes “the investigator(s)”; “you, your” – becomes “the participant(s)”
CONDITIONS
- Conditions/Focus of study are discrete and does not use verbs or complete sentences
- Keywords are not numbered or bulleted, each condition and keyword is listed individually, one per line
STUDY DESIGN
- All required fields are completed
- Verify Study Design based on protocol in IRB
- “Allocation” marked as “N/A” for single-arm studies
- Enrollment number Actual/Anticipated verified
ARMS/INTERVENTIONS
- Arm Title or Group/Cohort Label is brief and informative (even if there is only 1 arm)
- Interventions and intervention descriptions are entered correctly
- Arms/interventions are cross-referenced appropriately
OUTCOME MEASURES
- Title is specific and states WHAT is being measured
- only 1 variable must be assessed per outcome measure
- Description explains HOW outcome is being measured, not WHY it is being measured
- Scoring scale name, score range, significance of upper and lower limits specified (if applicable)
- Unit of measure specified
Time frame specified as a single time point or change between 2 time points
INCORRECT: “Safety and Toxicity”, Description: “Safety of study drug.”
CORRECT: “Safety as assessed by number of participants experiencing adverse events” Description: “Number of participants experiencing adverse events grade 3 or higher, as defined by Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0)”
ELIGIBILITY
- Age Limits are consistent with the Eligibility Criteria and with other parts of the record
- Eligibility criteria is divided into Inclusion/Exclusion criteria in bulleted format
CONTACTS/LOCATIONS
- Central Contact Person listed as a primary research team contact
- Study Officials: Person responsible for overall scientific leadership of the protocol, including the study Principal Investigator.
- Organization Affiliation: Full name of the Official’s organization (for UCI Researchers, its “University of California Irvine”)
- All study sites specified matches IRB
- Recruiting status for each study site accurate (if at least one study site is recruiting then Study Status reflects “Recruiting”)
- Each facility is listed in a separate field
IPD Sharing Statement
- Field is completed with a ‘Yes’ or ‘No’ selection
- If ‘Yes’ is selected, an IPD Sharing Plan is identified
- The Plan to Share IPD selection is consistent with the IPD Sharing Plan Description.
REFERENCES
- Each citation is listed in a separate field (if applicable)
- You will be prompted for Organization’s Unique Protocol ID.
- At UCI, the Unique Protocol ID MUST UCI IRB number (DIGITS ONLY no dashes, no #, same number as in KRP).
- Add the brief and official title for your study
- if using any acronyms- specify in the “Acronym” section
- Select Continue to complete the “Create New Record” module, and add information into each module of the protocol record as appropriate to your study.
- Secondary IDs:
- Grant‐funded projects MUST enter the sponsor‐issued grant or award number in this field. [1]
- For industry‐funded projects, use the sponsor’s protocol ID number
- For UCI Cancer Center studies, in the Secondary ID field, select "Other Identifier" and add the unique CFCCC identifier, with "Issuing Organization" as "UCI CFCCC".
- To ensure compliance with the requirements for each data entry field and module, refer frequently to the Definitions and Help links at the top of each module.
- Under the “Help” menu, go to the QUICK START GUIDE.
- Instructional links embedded throughout the PRS will aid users with navigation and record completion.
[1] U.S. National Institutes of Health (NIH) Grant/Contract Award Number: In the Secondary ID field, include activity code, institute code, and 6-digit serial number. Other components of the full award number (type code, support year, and suffix) are optional.
**EQUIP TIPS TO AVOID COMMON PRS ERRORS:
- Unique protocol ID is the IRB number ONLY (DIGITS only, no dashes, letters or #)
- Brief Title does not include study type (e.g., Phase I, Randomized…)
- Secondary IDs include NIH grant #s (verify in IRB Record) or CFCCC studies: Include additional a unique identifier
- The Record Verification Date reflects the last time the PRS record was updated. Revise this date each time the record is verified for accuracy and completeness.
- Complete the rest of the required information per guidance in this section:

- If unsure of dates, indicate the date as “anticipated” in the last dropdown under “Type”
- If recruitment has begun, specify the “actual” date the first subject was enrolled.
**EQUIP TIPS TO AVOID COMMON PRS ERRORS:
- Record Verification Date is the current month/year
- Overall Status matches IRB Status (in KRP)
- Completion Dates Actual/Anticipated have been evaluated for accuracy
- If timeframes for outcomes are the same, the primary and study completion dates are identical
- For all UCI Investigator Initiated studies, the Principal Investigator (Lead Researcher) should be designated as the Responsible Party (RP).
- Under Responsible Party choose “Principal Investigator” (PI)
- Select the Investigator name from the drop-down menu (email era@uci.edu if the PI does not have a PRS account)
- Enter the Investigator’s Official Title
- Investigator’s Affiliation should automatically populate to “University of California, Irvine”
- Sponsor: Regardless of funding source, enter the “regulatory sponsor” (primary organization overseeing the implementation of the study), usually University of California Irvine
- Any collaborating sites should be entered under the Collaborators section
- Click Continue
- U.S. FDA Regulated Drug / Device: Indicate whether this study involves an FDA‐regulated drug, biologic, or device.
- U.S. FDA Regulated Drug / Device: Indicate whether this study will be conducted with a drug/device product under a U.S. FDA Investigational New Drug (IND) Application or Investigational Device Exemption (IDE).
- Human Subjects Protection Review: Enter the current status of your IRB Protocol in KRP (e.g., “submitted, pending” if an application has been submitted but is under review.
- Add the following information for the UCI IRB:
- Board Name: University of California Irvine IRB
- Board Affiliation: University of California Irvine
- Board Contact: 949-824-8170
- Email: irb@uci.edu
- Business Address: 324 Aldrich Hall, Irvine, California, 92697-7600
- Data Monitoring: Indicate whether a data monitoring committee (board) has been appointed for this study
- Oversight Authorities: Name each national or international organization with authority over the protocol (e.g. DHHS, FDA, NIH, DOD, DOE, etc.)
**IMPORTANT: For studies that meet the criteria for clinical trials per NIH Definition and Applicable Clinical Trials, Consent / study information sheet contains requisite ct.gov language
**EQUIP TIP TO AVOID COMMON PRS ERRORS:
- IND/IDE information completed (if applicable)
- Enter all the applicable information regarding the study you are registering in the Study Description”, “Conditions”, “Study Design”, “Arms and Interventions”, “Eligibility” sections, as well as any other applicable sections.
- Review and reference the PRS User Guide
**Tip: Data is saved as each page is completed so that you can "Quit" at any time, saving the record to come back to and submit later**

- After filling in the last data entry page, the “Protocol Section” page appears with all of the information Review and “Entry Complete” if all information is complete.
- Address any ERROR messages if populated.
**GENERAL EQUIP TIPS TO AVOID COMMON PRS ERRORS:
- RECORD VERIFICATION DATE is current Month and year
- Unique Protocol ID is the IRB number ONLY (No other Unique protocol ID’s, IRB number in digits only, no dashes, #)
- Record Owner is the PI or Research Coordinator
- Contact info for Record Owner is up-to-date
- PI on record matches IRB PI
- NCT# included in IRB “Clinical Trials Information” section
- All Warnings/Errors addressed
- All parenthetical citations have been removed
- All acronyms have been expanded on their first use
- Spell-check complete
- Free-text fields are blank if there is no information to report, and do not contain text such as “TBD,” “Pending,” “N/A,” “None”
- If the PI is the responsible party, they will review all entries made by study staff prior to release.
- Once the information is confirmed (passed internal review), under “Record Status” – click “Approve”:

- Once the information is confirmed (passed internal review), under “Record Status” – click “Approve”:
- Select “Release” to submit the record to ClinicalTrials.gov. The responsible party confirms their identity, and that the record is up to date, reviewed for accuracy and completeness:
- Once released, PRS staff perform final review and processing of the record.

- Note: some records may receive PRS Review Comments that identify major issues that must be addressed by the responsible party before the record will be made available on ClinicalTrials.gov.
- Following successful PRS review, records are made available to the public through the ClinicalTrials.gov web site within 2 to 5 days of Release.
- The ClinicalTrials.gov Identifier (NCT number) is assigned as part of that process.
- Please download and review the IRB EQUIP TIPS guidance document called “CHECKLIST TO ADDRESS COMMON PRS ERRORS” to avoid common issues noted by the ClinicalTrials.gov database (PRS) staff prior to public release.
- Directly email PRS staff if you have any questions or need help related to PRS Review Comments at register@clinicaltrials.gov
- Once a record is created, you can update it as long as it is not undergoing PRS review:
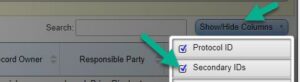
- If your department is utilizing the “Secondary ID” Section for a Unique department ID, click “Show/Hide Columns” next to the search bar in PRS, and select “Secondary IDs” as part of your records filter:
When / how often must I update ClinicalTrials.gov registration information?
- The Record Verification Date should be updated any time the responsible party reviews the complete set of submitted clinical trial information for accuracy and not less than every 12 months, even if no other updated information is submitted at that time.
- Responsible Parties should review the Clinical Trial Registration Data Elements for More Frequent Updating Table as many elements must be updated within 30 days of a change.
- It is recommended that the Record Verification Date be updated at least every 6 months for studies that are not yet completed, even if there were no changes to the record.
Clinical Trial Registration Data Elements for More Frequent Updating
| Data Element | Deadline for Updating (i.e., not later than the specified date) |
|---|---|
| Study Start Date | 30 calendar days after the first subject is enrolled (if the first human subject was not enrolled at the time of registration). |
| Intervention Name(s) | 30 calendar days after a nonproprietary name is established. |
| Availability of Expanded Access | 30 calendar days after expanded access becomes available (if available after registration); and 30 calendar days after an NCT number is assigned to a newly created expanded access record. [1] |
| Expanded Access Status | 30 calendar days after a change in the availability of expanded access. |
| Expanded Access Type | 30 calendar days after a change in the type(s) of available expanded access. |
| Overall Recruitment Status | 30 calendar days after a change in overall recruitment status. [2] |
| Individual Site Status | 30 calendar days after a change in status of any individual site. |
| Human Subjects Protection Review Board Status | 30 calendar days after a change in status. |
| Primary Completion Date | 30 calendar days after the clinical trial reaches its actual primary completion date. |
| Enrollment | At the time the primary completion date is changed to "actual," the actual number of participants enrolled must be submitted. |
| Study Completion Date | 30 calendar days after the clinical trial reaches its actual study completion date. |
| Responsible Party, by Official Title | 30 calendar days after a change in the responsible party or the official title of the responsible party. |
| Responsible Party Contact Information | 30 calendar days after a change in the responsible party or the contact information for the responsible party. |
| Device Product Not Approved or Cleared by U.S. FDA | 15 calendar days after a change in approval or clearance status has occurred. |
| Record Verification Date | Any time the responsible party reviews the complete set of submitted clinical trial information for accuracy and not less than every 12 months, even if no other updated information is submitted at that time. |
Want to Avoid Common Errors?
Use this checklist!
Reporting Results
Reporting Results
Who is responsible for reporting results?
The Principal Investigator is the Responsible Party that must publish the results on ClinicalTrials.gov.
Walkthrough Webinar
What studies need to report results?
The Food and Drug Administration (FDA) and National Institutes of Health (NIH) require the publication of results for certain studies on ClinicalTrials.gov (Ct.gov).
-
-
- See FDAAA 801 and the Final Rule for more information.
- See How to Submit Your Results for details.
-
The ICMJE Policy recommends results publication but it is not required.
When is results reporting required?
Submission of results information is required no later than 12 months after the Primary Completion Date (the last subject last visit) of the clinical trial, which is defined as the date of final data collection for the primary outcome measure.
How to complete the results section?
| RECORD SECTION | TIPS |
|---|---|
| PARTICIPANT FLOW | Protocol Enrollment refers to total number of subjects who consented to protocol (including screen failures, withdrawals, etc.) |
| Recruitment details (optional) explains any specifics used at time of recruitment | |
| Pre-assignment details explains (in detail) what happened to subjects who signed consent but were not assigned to an arm/intervention (i.e., how many screen failures, withdrawals, etc.) | |
| Arms and arm descriptions specified consistent with protocol section | |
| Number of Participants Started refers to total number of participants assigned to each arm | |
| Number of Participants Completed refers to total number of participants who completed study intervention | |
| Reason(s) for Not Completed provided | |
| Divided into periods/milestones appropriately | |
| Total number of participants started cannot be greater than enrollment number | |
| BASELINE CHARACTERISTICS | Overall Number of Baseline Participants should match Number of participants Started (from Participant Flow) |
| Baseline Analysis Population Description explains if there is a discrepancy between Overall & Started numbers | |
| Arm titles/descriptions are consistent with participant flow and/or protocol section | |
| Data is presented per arm | |
| If “number of participants” is reported, make sure Measure Type is “Count of Participants” | |
| Measure description is specified for all Study-specific measures | |
| OUTCOME MEASURES | Titles/descriptions/time frame meet the criteria (as specified on prior checklist) |
| Results are reported per arm | |
| Population Analysis Description includes reason why Number of Participants analyzed is different than total number of participants completed (if applicable) | |
| Type and Number of Units analyzed is indicated, if other than “number of participants” (i.e., # of Lesions) | |
| Unit of measure matches what is stated in Outcome Title/Description | |
| Sum of all results entered for each arm equals overall number of participants analyzed | |
| Verify true data is entered and there are no placeholders | |
| Statistical Analysis portion is completed | |
| ADVERSE EVENTS | Time frame specified |
| Collection Approach specified | |
| Arm titles/descriptions consistent with other sections in the record | |
| Data presented per arm | |
| All-cause mortality specified (cross-check with number “not completed due to death” from participant flow and any mortality measures in outcome section, if applicable) | |
| Total Number “At Risk” must be equal to total number of participants who started the study | |
| CERTAIN AGREEMENTS | Disclosure restrictions should be ‘No’ unless documentation is presented to the contrary |
| RESULTS POINT OF CONTACT | Responsible Party's Contact information will be public facing |
| Information is correct and valid email address/phone number entered | |
| DOCUMENT SECTION | Documents in pdf/a format |
| Protocol (required for primary completion date after January 18, 2017) | |
| Statistical Plan (required for primary completion date after January 18, 2017) |
Posting the Consent Form
Posting the Consent Form
All clinical trials funded by an agency that has signed onto the Common Rule initially approved on or after January 21, 2019 OR that transitioned to the 2018 Common Rule Requirements (45 CFR 46.101(l)) and the transition determination was documented and dated by the IRB before the trial is closed to recruitment and 60 or fewer days before the last protocol-required study visit by any subject enrolled in the protocol.
OHRP Guidance
Posting the Consent Form
WHAT TO POST
One IRB-approved consent form used to enroll subjects. Even if multiple versions were approved by the IRB, only one must be uploaded.
WHEN TO POST
After the clinical trial is closed to recruitment, and no later than 60 days after the last study visit by any subject, as required by the protocol.
WHERE TO POST
Two federal websites have been identified as satisfying 45 CFR46.116(h): ClinicalTrials.gov and a designated docket folder on Regulations.gov (Docket ID: HHS-OPHS-2018-0021).
ClinicalTrials.gov FAQs
Burden of time: Per DHHS 42 CFR Part 11, the initial submission of registration information will take an average of 8 hours, with each update requiring 2 hours apiece and will take place 8 times during the course of the study (an average of 32 hours!).
DHHS expects that voluntary registrations will:
- submit the same clinical trial registration information as applicable clinical trials.
- expects that required elements of the record are updated as frequently as information for applicable clinical trials.
Voluntary registration places a burden on the Responsible Party to comply with all registration and update requirements. Any ongoing non-compliance with these requirements is on the part of the Responsible Party.
| Name | Type | Intervention Type | Registration Policy Scope | Results Submission Policy Scope |
|---|---|---|---|---|
| Final Rule for Clinical Trials Registration and Results Information Submission (42 CFR Part 11) | U.S. Federal regulation implementing FDAAA 801 effective in 2017 | Drug, biological, and device products | Clinical trials of a Food and Drug Administration (FDA)-regulated drug, biological, or device product other than Phase 1 (drug/biological products) or small feasibility studies (device products) | Same scope as registration |
| NIH Policy on the Dissemination of NIH-funded Clinical Trial Information | National Institutes of Health (NIH) policy effective in 2017 | Any (includes drug, biological, and device products, as well as surgical procedures, and behavioral interventions) | Clinical trials funded in whole or in part by NIH | Same scope as registration |
| International Committee of Medical Journal Editors (ICMJE) Policy | Publication policy initiated by the International Committee of Medical Journal Editors (ICMJE) in 2004 | Any (includes drugs, biological, and device products, as well as surgical procedures, and behavioral treatments) | All interventional studies, including Phase 1 studies; defines criteria for "acceptable registries" | The ICMJE expects authors to meet results information submission requirements "of their funding and regulatory agencies" and "encourages... results reporting even when not required." |
 The “Responsible Party” refers to the entity or individual who is responsible for registering a trial on ClinicalTrials.gov.
The “Responsible Party” refers to the entity or individual who is responsible for registering a trial on ClinicalTrials.gov.- The Responsible Party is responsible for the initial release of the record, all future updates and ensuring the trial registration stays accurate and up-to-date.
- For studies registered by UCI, the PI serves as the responsible party if they meet all the following criteria:
-
- They are responsible for conducting the clinical trial.
- They have access to and control over the data
- They have the right to publish the results of the trial
- They can meet all the requirements for the submission of clinical trial information.

- A ClinicalTrials.gov staff member will review the study record after it is submitted and before it is published on ClinicalTrials.gov.
- The review process may take up to a few days. Ensuring that the record is consistent with the ClinicalTrials.gov Protocol Review Criteria (PDF) before releasing it will expedite publication on the site.
- After it is accepted by review staff for publication, the record, including its NCT Number, will be available on ClinicalTrials.gov within 2–5 business days.
The date of registration is the date The date on which the study sponsor or investigator first submitted a study record to ClinicalTrials.gov. A trial is registered in ClinicalTrials.gov after a new “protocol record” is created and passes QA review in the Protocol Registration and Results System (PRS) for ClinicalTrials.gov. Once a record passes review with the PRS reviewers, it is assigned a clinical trial identifier (NCT#) and registered.
Required Registration Updates
See related FAQ on the ClinicalTrials.gov related webpage
Responsible Parties should update their records within 30 days of a change to any of the following:
- Recruitment Status (1) and Overall Recruitment Status data elements on ClinicalTrials.gov
- Completion Date (See Primary Completion Date data element on ClinicalTrials.gov)
Other changes or updates to the record, such as protocol amendments, must be made at least every 12 months.
**It is recommended that the Record Verification Date be updated at least every 6 months for studies that are not yet completed, even if there were no changes to the record.
Results Reporting:
- The Food and Drug Administration Amendments Act (FDAAA), National Institutes of Health (NIH) require the publication of results for certain studies on ClinicalTrials.gov (Ct.gov).
- See FDAAA 801 and the Final Rule for more information.
- See How to Submit Your Results for details.
- The ICMJE Policy recommends results publication but it is not required.
- After a clinical trial has been registered on ClinicalTrials.gov and the study is completed, the Responsible Party must publish the results on ClinicalTrials.gov.
- Submission of results information is required no later than 12 months after the Primary Completion Date (the last subject last visit) of the clinical trial, which is defined as the date of final data collection for the primary outcome measure.
1 - If Overall Recruitment Status is changed to "suspended," "terminated," or "withdrawn," the Why Study Stopped data element must be submitted at the time the update is made.
If needed submit an Amendment for the following
- For studies that meet the criteria for clinical trials per NIH Definition and Applicable Clinical Trials, ensure the ClinicalTrials.gov statement is included in the Consent Form / Study Information Sheet
- Under “Type of Research” – “yes” to Does this research meet the definition of a clinical trial that requires adherence to Clinicaltrials.gov?
- Once obtained, NCT# is added to this section
- Recruitment section reflects ClinicalTrials.gov as a recruitment method.
Please see the Clinicaltrials.gov related FAQ
- For ACTs that are required by 42 CFR 11.22(a) to be registered and with a primary completion date on or after January 18, 2017:
- The responsible party's obligation to submit updates under 42 CFR 11.64(a) ends when all required clinical trial results information has been submitted as specified in 42 CFR 11.48 and the responsible party has made all corrections and/or addressed all concerns in response to any electronic notice received under 42 CFR 11.64(b)(1).
- For ACTs that are required by 42 CFR 11.22(a) to be registered and with a primary completion date before January 18, 2017:
- If the ACT studies a drug, biological, or device product that is approved, licensed, or cleared as of the primary completion date, then the responsible party's obligation to submit updates under section 402(j)(4)(C) of the Public Health Service Act (PHS Act) ends when all required clinical trial results information has been submitted as specified in sections 402(j)(3)(C) and 402(j)(3)(I) of the PHS Act.
- If the ACT studies a drug, biological, or device product that is not approved, licensed, or cleared for any use as of the primary completion date, then clinical trial results information must be submitted not later than 30 days after approval, licensure, or clearance of the drug, biological, or device product for any use, in accordance with section 402(j)(3)(E)(iv) of the PHS Act and pursuant to the Federal Court decision in Seife et al. v. HHS et al., 18-cv-11462 (NRB) (S.D.N.Y. Feb. 24, 2020). A responsible party's obligation to submit updates for such ACT under section 402(j)(4)(C) of the PHS Act ends when all required clinical trial results information has been submitted as specified in sections 402(j)(3)(C) and 402(j)(3)(I) of the PHS Act.
- For clinical trials initiated on or after January 18, 2017, for which clinical trial information is submitted voluntarily pursuant to 42 CFR 11.60(c):
- The responsible party's obligation to submit updates under 42 CFR 11.60(c)(2)(v), 11.64(a)(1)(ii), and 11.64 (a)(2) ends when all required clinical trial results information has been submitted as specified in 42 CFR 11.48(a) and the responsible party has made all corrections and/or addressed all concerns in response to any electronic notice received under 42 CFR 11.64(b)(1).
| Entity | Registration | Results Reporting | Penalties |
|---|---|---|---|
| Health and Human Services (HHS) | Within 21 days of enrollment | Within 365 days of primary completion date for ACTs | |
| National Institutes of Health (NIH) | Within 21 days of enrollment | Within 365 days of primary completion date for clinical trials receiving NIH funding |
|
| National Cancer Institute (NCI) | Within 21 days of enrollment | Within 365 days of primary completion date of NCI-supported clinical trials (in a peer-reviewed journal and/or ClinicalTrials.gov) |
|
| Veterans Health Administration (VHA) | Prior to release of funding. Prior to enrollment | Within 365 days of primary completion date |
|
| Centers for Medicare & Medicaid Services (CMS) | All qualifying clinical trials | Study-specific |
|
| Patient-Centered Outcomes Research Institute (PCORI) | All Clinical studies (including observational) | Expected of all PCORI Clinical studies – 500 word abstract published on PCORI website |
|
| International Committee of Medical Journal Editors (ICMJE) | Prior to enrollment |
|
|
| Department of Defense (DoD) | Prior to enrollment. Prior to release of funding. | Study-specific |
|
Adapted from: Clinicaltrials.gov enforcement: an update. Anthony Keyes, John Hopkins University. January 18, 2022
Why Do I Need to Register My Trial and Submit Results to ClinicalTrials.gov? Here are just some of the reasons…
- Required by Law: Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801) requires responsible parties to register clinical trials and submit summary results to ClinicalTrials.gov.
- Required for Journal Publication: The International Committee of Medical Journal Editors (ICMJE) requires trial registration as a condition of the publication of research results generated by a clinical trial.
- All NIH-funded clinical trials are expected to register and submit results information to Clinicaltrials.gov, as per the "NIH Policy on Dissemination of NIH-Funded Clinical Trial Information" for competing applications and contract proposals.
- WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects: "Every research study involving human subjects must be registered in a publicly accessible database before recruitment of the first subject" (para. 35).
What is the penalty for non-compliance?

Under NIH Policy: Noncompliance with the terms and conditions of the NIH award may provide a basis for enforcement actions, such as withholding current and future funding [45 CFR 75.371, 42 CFR 11.66]
Under Final Rule, responsible parties could be held accountable for noncompliance, with the potential for substantial civil monetary penalties, the withholding of grant funding from HHS agencies, and criminal proceedings.[2016 NEJM article]
- Case in point:
On 28 April 2021, the FDA issued its first Notice of Noncompliance to Georgia-based Accuitis who failed to submit required summary results information. The company could be “subject to a civil monetary penalty of $10,000 for each day of the violation” until the noncompliance is corrected. - FDA has issued already issued multiple Notices of Non-Compliance
- In addition, as of 12/04/2023 the FDA began publicly posting Pre-Notices for Potential Noncompliance
- This means that even if the responsible party resolves it so a Notice of Noncompliance is never sent or posted, the Pre-Notice for Potential Noncompliance and the responsible party will be publicly posted!
ICMJE: authors failing to prospectively register a trial risk its inadmissibility to journals following the ICMJE’s trial registration policy.
If the Responsible Party has joined / will leave UCI, PRS and UCI EQUIP Staff can assist in transferring a record to another PRS account. Records are registered under their sponsoring organization's account. Therefore, a sponsored study must stay in the account of its sponsor even if/when an associated investigator leaves that institution.
A UCI PRS Administrator will request the transfer by emailing PRS Staff at register@clinicaltrials.gov. To request a PRS record transfer, please email EQUIP staff at EQUIP@uci.edu.
Transfers must be coordinated between the organizations involved. The receiving organization PRS Administrators are responsible for coordinating the transfer. For organizations without Administrators, the Responsible Party and Record Owners must coordinate the transfer.
To complete a record transfer, PRS Staff must receive:
- Confirmation from the receiving organization or Responsible Party that the record will be accepted (a copy of email confirmation is acceptable)
- Name of the receiving organization
- Username of the new Record Owner
- NCT number of the record
Note: Records in a Released state cannot be transferred.
ClinicalTrials.gov Office Hours
FRIDAYS BY EMAIL REQUEST
Anu Mathur
Anuradhm@uci.edu
Out of Office: June 21 through July 6
EQUIP Manager