KR Proposal Development
The KRPD user guide has been divided into two versions, one tailored for Initiators & Faculty and the other for Officers. Please choose the version most appropriate for you, using the toggle below. The default is Initiators & Faculty.
Intro to KR Proposal Development for Faculty & Intitiators
Logging In
- Go to Zot!Portal (portal.uci.edu).
- Click Login in the upper right corner.
- Enter your UCInetID and password in the Web Authorization screen
- Click Login.
- Navigate to Faculty & Staff > Research > Kuali Research Proposals.
- Click Create a Proposal.
- If the Research Tab is not available to you, please contact the ERA Team.
If you receive an error message in the KR system that you are not authorized to initiate Proposal Development Document, or you do not have access to create a proposal for your Lead Unit, contact your Department DSA to grant access to the Proposal Creator and/or Administrative Contact roles in KSAMS. If you need help determining who your Department DSA is, please contact the ERA Team.
- Proposal Creator KSAMS role will allow you to initiate, view, edit, and submit proposals for a lead unit.
- Administrative Contact KSAMS role will allow you to view, edit, and submit proposals for a lead unit.
To grant access to a specific proposal (but not globally for a lead unit), refer to Access instructions in the Creating a Proposal section.

Starting a Proposal
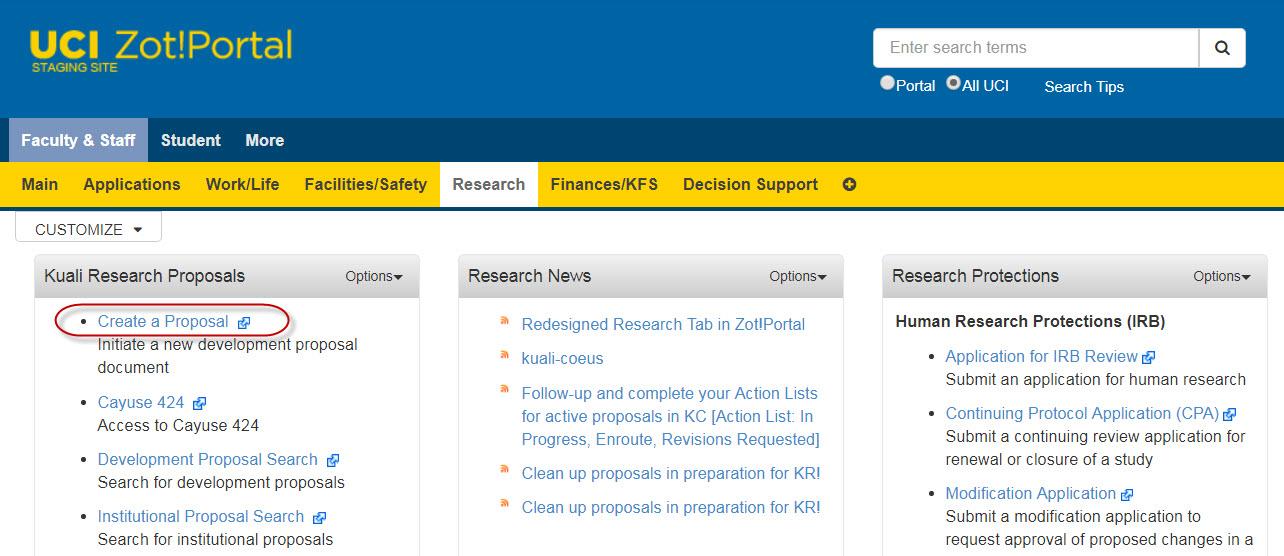
Upon clicking Create a Proposal, you will be taken to a new page that collects information that is required to start a proposal document. Having all the required fields completed allows the Proposal Development record to be saved and recorded in the KR system. In the ‘Create Proposal’ process, when the initial required fields for saving fields are completed and the user clicks ‘save and complete,’ the Proposal Details screen appears.

Jump to the Creating a Proposal section in this user guide to continue completing the Proposal Development Document. Or, continue to the Navigating a KR Proposal section for more information about the KR Proposal interface and description of reference information, action links and buttons.
Header
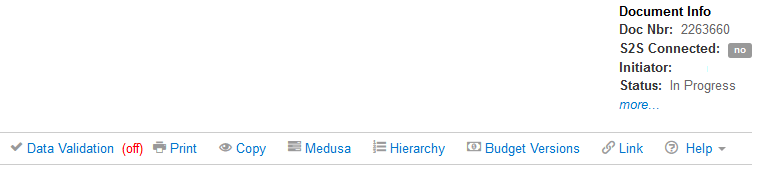
The document header on the top displays six fields of reference information about the Proposal document.

| Left - Proposal Info | Description |
|---|---|
| Proposal number | Number incrementally assigned by the system |
| Principal Investigator (PI) | Not yet assigned displays until a PI is added on the Key Personnel screen, then the person’s name is displayed. |
| Right – Document Info | Description |
| Doc Number | Numberincrementally assigned by the system |
| S2S Connected | Status indicated by a gray ‘no’ box until a successful S2S Opportunity is applied and is updated to a green ‘yes’ box. *Note: UCI is not currently utilizing Kuali S2S. |
| Status | Submission state of the proposal (In Progress, Enroute, Approved, Submitted, etc.) |
| ‘more’ | Click to display information expanded from the ‘doc info’ data including the create and last update timestamps as well as the sponsor name. |
Proposal Toolbar
The Proposal Toolbar provides access to Proposal Actions.
| Horizontal Band Toolbar | Description |
|---|---|
When (off): click to open the Data Validation options window and turn validations (On) When (on); click to turn off the validations, to view the list of existing validations, their location, description, severity (warning or error), and the ‘fix’ button to navigate directly to the validation location |
|
| Click to open the available print options: Sponsor Form Packages; or the Reports (Current Support, and Pending Support) | |
| Copy | Click to open the Copy Proposal window |
| Medusa | Click to view the relationship this proposal has to other KC documents |
| Hiearchy | Click to open the Proposal Hierarchy builder window. Use this tool to link the current proposal to an existing hierarchy, or to create a new hierarchy. (See section on Hierarchy) |
| Budget Versions | Click to open a Budget Version window to add a new budget version, open an existing budget, or use one of the Action options (View Summary, Copy (budget), Print (reports), or Submit (mark the version as the final/complete to submit with this proposal) |
| Link | Click to obtain a direct link to the Proposal you are currently in |
| Help | When the Help link is clicked it will expand a dropdown that has selections of different areas in the proposal. Upon clicking one of the options it will open a new browser window linked to either a Kuali Research article (default) or a custom linked site configured via the below listed parameters |
Creating a Proposal (The Basics)
Proposal Details
Proposal Details is the first screen in the Basics menu navigation of the proposal. In the ‘Create Proposal’ process, when the initial ‘required fields for saving’ fields are completed and the user clicks ‘save and complete,’ the Proposal Details screen appears. When opening existing proposals, the Proposal Details screen is the opening screen.
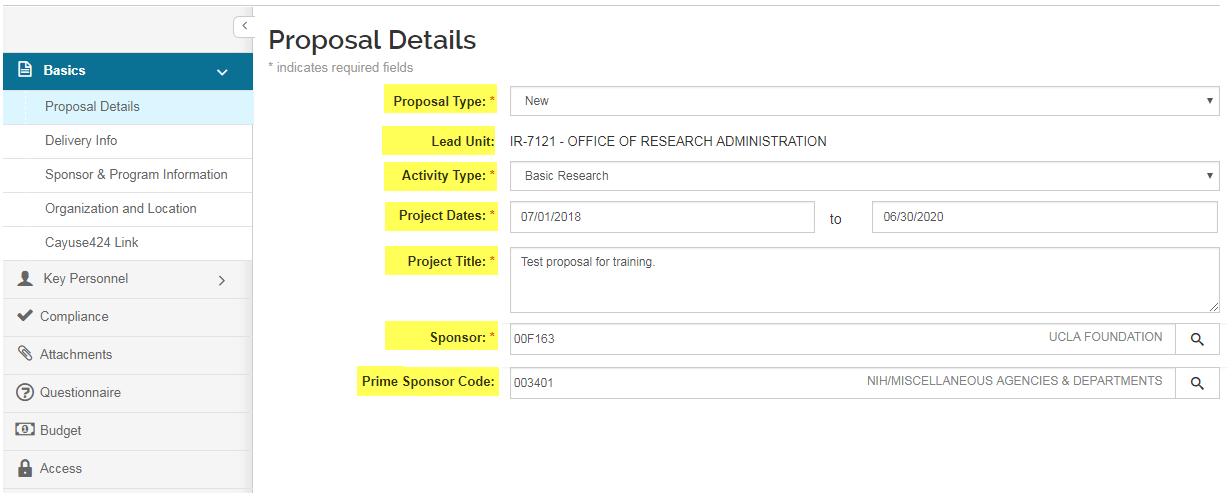
Required fields for saving (those marked with an asterisk *) may be edited once the proposal has been saved. To edit or enter into the field you have the following field types:
- Drop-Down: scroll through the options and highlight your selection
- Date: click in the field to activate the calendar tool (either use the tool or manually enter date)
- Text: click in the field and edit the existing text
- Lookup: click in the field and use the lookup tool to locate the item
The proposal will remain editable while saved but once submitted into routing for approval it will be locked down. If the proposal is returned to the initiator for edit during the workflow review, it will become editable again.
- Proposal type - Used to identify the type of proposal (New, Continuation, Renewal or Supplement).
- Lead Unit - The unit/department assigned to a proposal. (Proposal Creator Access is managed in KSAMS. Refer to the Logging In section for instructions on obtaining Proposal Creator and/or Admin Contact role access in KSAMS.)
- Activity Type - The university functional area for this proposal.
- Basic Research
Research that is directed toward increase of knowledge in science wherein the primary aim of the investigator is a fuller knowledge or understanding of the subject under study, rather than a clear or direct practical application thereof. This includes analytical and experimental activities that primarily seek to increase the understanding of fundamental phenomena. The end product is usually a report, although experimental hardware may be involved. In basic research, the particular use of the knowledge is not foreseen or identified at the beginning of the effort. - Applied Research
Consists of the effort that: 1) normally follows basic research, but may not be severable from the related basic research; 2) attempts to determine and expand the potentialities of new scientific discoveries or improvements in technology, materials, processes, methods, devices, and techniques; and 3) attempts to "advance the state of the art". Applied research involves the study of phenomena relating to specific, known needs in connection with the functional characteristics of a system. Applied research does not include any efforts where the principal aim is the design, development, or test of specific articles or services to be offered for sale, which are within the definition of the term development. - Developmental Research
The systematic use and practical application of investigative findings and theories of a scientific or technical nature toward the production of, or improvements in, useful products to meet specific performance requirements, but exclusive of manufacturing and production engineering. The dominant characteristic is that the effort be pointed toward specific problem areas to develop and evaluate the feasibility and practicability of proposed solutions and determine their parameters. Development includes studies, investigations, initial hardware development and ultimately development of hardware, systems or other means for experimental or operational test. - Other Research
Used only if a research project cannot be classified as basic, applied, developmental, or clinical trial research. - Training
For the purposes of reporting to Office of the President, this category means the conduct of scholarly, professional or occupational instruction for matriculated students or University employees in forms such as classes, seminars, workshops, conferences, etc. This category includes sponsorship of students or employees who are "in training" primarily but not exclusively at the graduate and postgraduate levels. The scope of this code also includes sponsored training awards made to The Regents which provide for selection of student recipients by academic departments, and the institutional support which is either included in the training grant or is applied for and awarded separately. Excluded from this group and from this contract and grant information requirement are fellowships or other similar awards made directly from sponsors to students, and Student Aid programs.
Awards and proposals that require both the development of training materials and the conduct of training as part of the same award should be identified as Training, (Category 5).
Awards and proposals that are primarily for development of training materials and curricula should be identified as Developmental Research, (Category 3).
Training projects that are intended for the training to be conducted by the University for presentation to and for primary benefit to the public, i.e., individuals or groups external to the University, should be identified as Public Service, (Category 6). - Other Sponsored Activity
Programs and projects financed by Federal and non Federal agencies and organizations which involve the performance of work other than instruction and organized research. Examples of such programs and projects are health service projects, and community service programs. However, when any of these activities are undertaken by the institution without outside support, they may be classified as other institutional activities. - Equipment
Applications or awards that are restricted by the sponsor for the sole purpose of the University's procurement of equipment. This may include direct grants of equipment, or full or partial funding to enable the University to purchase equipment, where in both cases the sponsor intends to transfer or have title to the equipment vest in the University. - Public Service
As the term is interpreted in the context of sponsored projects, Public Service means externally sponsored projects where the sponsor, particularly the Federal and state Government, desire to have the University provide the benefits of scholarly or professional training or services to individuals or sponsor-designated recipient groups that are external to the University. Examples of public service may include programs such as those sponsored by the Agency for International Development, the National Endowment for the Humanities and the National Institutes of Health in the area of biomedical services or training. The principal characteristic of public service is that individuals and groups external to the University are the intended beneficiaries. - Clinical Trial Research
*For purposes of applying 32% F&A rate, activity must meet this definition:
The controlled, clinical testing in human subjects of investigational new drugs, devices, treatments or diagnostics, or comparisons of approved drugs, devices, treatments or diagnostics, to assess their safety, efficacy, benefits, costs, adverse reactions, and/or outcomes.
Such studies may be conducted under an industry-developed protocol or an investigator-developed protocol. These studies are most often conducted in conjunction with obtaining new drug (under Phase I, II, III, or IV) or device approval from the U.S. Food and Drug Administration, although they can be designed with the sole purpose of collecting and analyzing data about approved drugs or devices in order to contribute to medical knowledge about the treatment of a disease or medical condition. In all cases, the study must include the prospective enrollment of human subjects and the controlled testing of a drug, device, or diagnostic under an approved protocol.
Federally-funded projects, projects funded by interest groups, charities or the State of California, retrospective chart reviews, analysis of existing medical data and records, laboratory research, and animal studies are not categorized as clinical trials for purposes of applying the clinical trial F&A cost rate. - Clinical Research
Clinical research is any research involving human subjects, even if Institutional Review Board (IRB) approval is not required, which fits into any of the three following categories:- Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. (non-CT)
- Medical records research. Medical records research involves the use of information collected from medical records, including imaging data, and blood and tissue repositories. (non-CT)
- Other
To be used only if a project can not be classified by one of the above categories. - Fellowship
An award made it to the University to support the research experience of an individual, usually a graduate student or postdoctoral scholar, where the individual is not acting as an employee of the University, but rather as a fellow earning a subsistence stipend as part of the award. The award may also included funding for research supplies. Fellowship awards do not fund equipment, faculty salaries or F&A expenditures.
Primary characteristics of fellowship:- Subsistence for research experience
- Typically for student
- No employer-employee relationship
- Basic Research
- Project Dates - Beginning and end of the entire project period.
- Project Title - Proposal project title.
- Sponsor - A unique identifier for the sponsoring organization for the project. Kuali will display the Sponsor Name to the right of the Sponsor Code. You can either begin typing in the name of the sponsor, or use the magnifying glass lookup tool to search for the sponsor.
*Can't find or need to request a new sponsor code? Please contact the ERA Team for assistance.
- Prime Sponsor Code - A unique identifier for the prime sponsoring organization, which is the original funding source for the project. Kuali will display the Prime Sponsor Name to the right of the Prime Sponsor Code. Complete this field if you will not submit this proposal directly to the funding agency, but to a collaborator for inclusion in their application to the funding agency. (e.g. you are submitting as a subawardee).
Delivery Info
The Delivery Info screen is optional and can be used to provide information to SPA. This screen is relevant for submissions that need cover letters signed by the authorized official, and proposals that will be submitted directly to the sponsor by the PI.
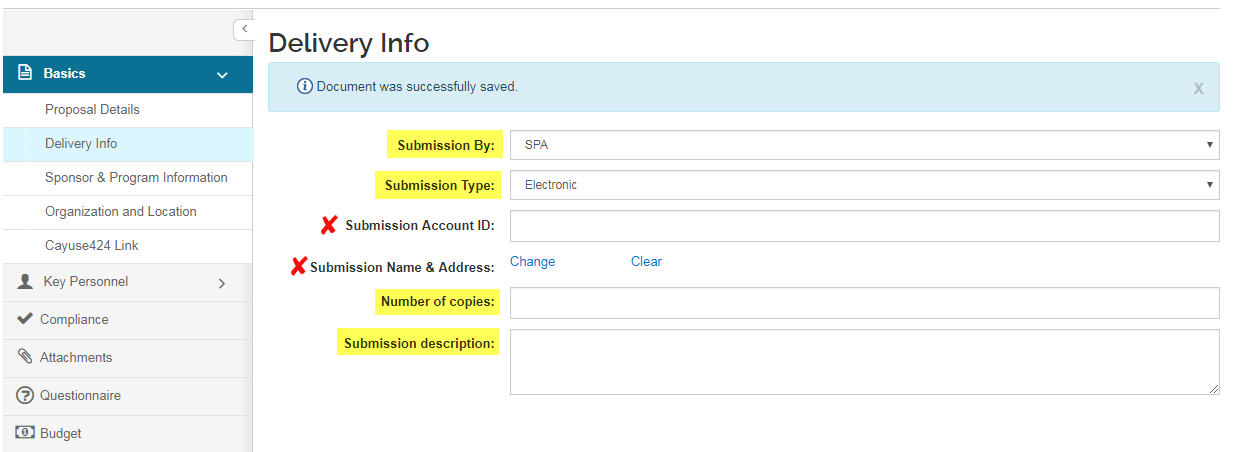
- Submission By: Select an option to describe which party is responsible for submitting the authorized proposal to the sponsor
- Submission Type: Select an option to describe in how the proposal will be transmitted
- Submission Account ID: Leave blank.
- Submission Name & Address: Not required.
- Number of Copies: if applicable, enter a numeric value for the number of proposal hardcopies that must be provided or submitted.
- Submission Description: use this field to provide information and details about the submission.
Sponsor and Program Information
This screen is used to provide submission information regarding the proposal.
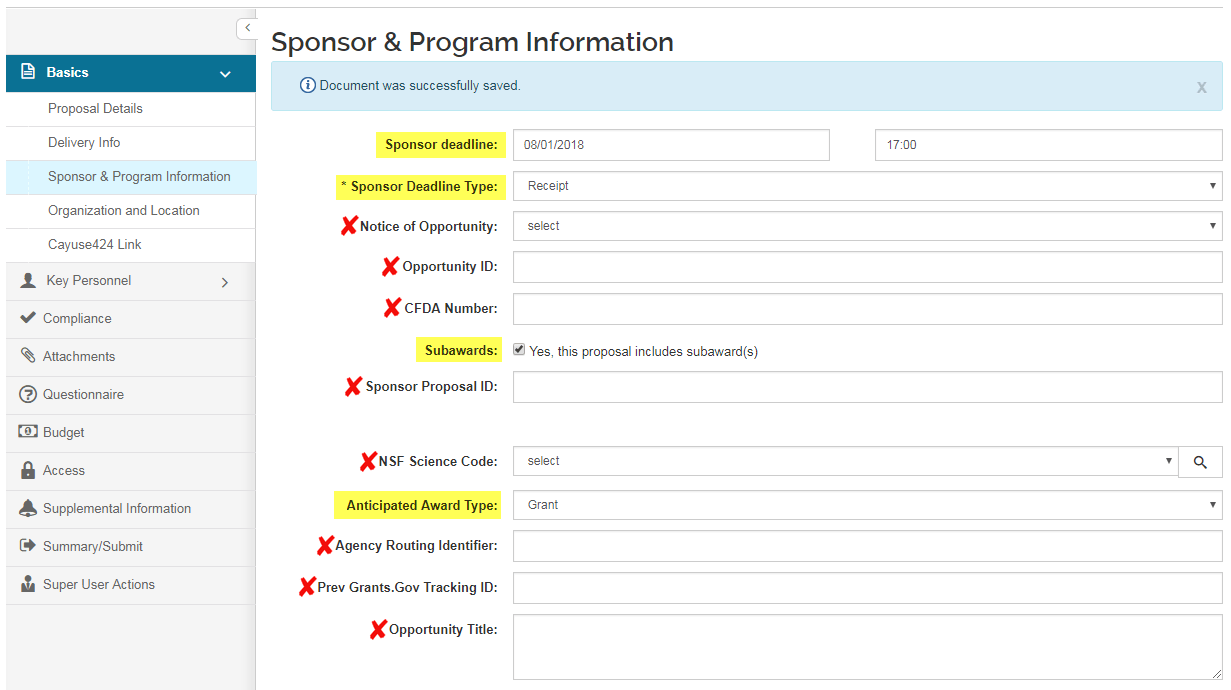
- Sponsor Deadline (date) - Click in the field to open the calendar tool to select the date, or type in mm/dd/yyyy format to populate the box with the date the proposal is due to the sponsoring agency
- Sponsor Deadline (time) - Optional. Click in the field to enter the time of day that the proposal is due at the sponsoring agency in ‘hh:mm’ format using military time format e.g. for 5:00PM, enter 17:00
- Sponsor Deadline Type - Click in the field to view the available options: scroll to select one. (Postmark, Receipt, Target)
- Notice of Opportunity - Leave blank.
- Opportunity ID - Leave blank.
- CFDA Number - Leave blank.
- Subawards - Check the box to indicate whether the proposal includes subawards to other institution/entities.
- Please see the Subawards section in the Additional Information section below for instructions on how to include subaward information in your proposal document.
- Sponsor Proposal ID - Leave blank.
- NSF Science Code - Leave blank.
- Anticipated Award Type - Click within the field to display the list, and then click on an item in the list to highlight and select it to make your selection.
- Opportunity Title - Leave blank.
Organization and Location
Information regarding the Performance Site location is no longer required. Information regarding whether the project will be conducted On-Campus or Off-Campus will now be included on the Supplemental Information section.
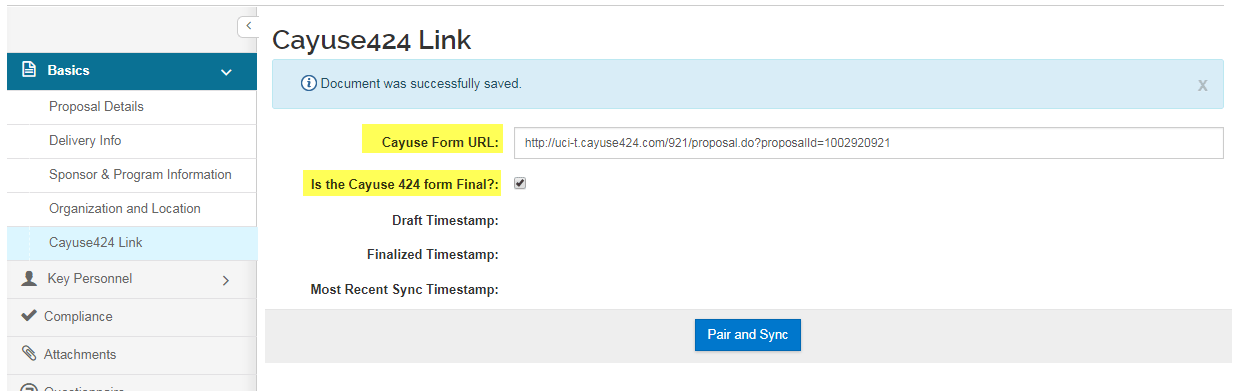
Cayuse 424 Link
For grant applications to Federal sponsors utilizing Cayuse 424, paste the link to the Cayuse proposal here. When the proposal is final and ready for submission, check the box to indicate the that it is final.
To sync information from Cayuse to KR (e.g.: project title & dates, personnel), click on the "Pair and Sync" button. Confirm that you would like to sync these data elements. The draft timestamp and most recent sync timestamp will appear.
Key Personnel
This screen is used to add Principal Investigators (PI), any additional Multiple-PI’s (MPI) and Key Persons (proposal role description required) to the proposal.
*Use the Investigator role for all individuals who meet the COI definition of an Investigator for PHS/DOE/NSF proposals and are not considered a Key Person.

The Add Personnel button will open the lookup where you can search/return an individual, define their role, and then add to the proposal.
Click Add Personnel to open the person search window.
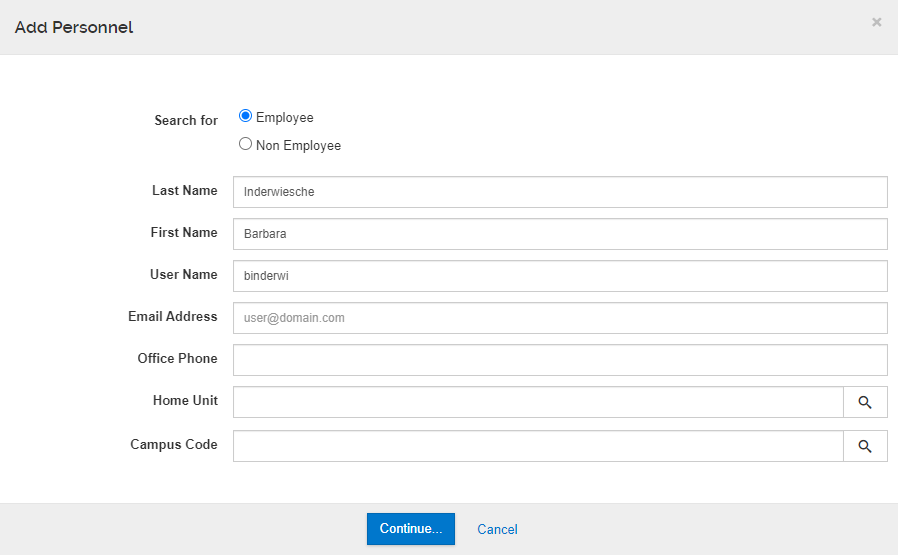
Click Add Personnel to open the person search window.
1. Ensure "Employee" is selected - it should be the default selection.
2. Enter search criteria to find the person you are looking for; use several fields in the lookup to limit the search results. User Name is their UCI Net ID.
3. Click Continue to proceed with the search.

4. Review search results and click the radio button at the row you want to select; click Continue.
- Or click ‘Go Back’ to perform another search.
- Or click the ‘x’ button in the upper right of the window to exit and close the search.

5. With a selected person, Assign a role by clicking a radio button beside one of the selections: Principal Investigator or PI/Contact, PI/Multiple, Co-PI, Key Person or Investigator
- Principal Investigator is selected by default for the initial search, and any subsequent search until that role is assigned.
- PI/Multiple will only appear if the proposal is a PHS/NIH sponsor.
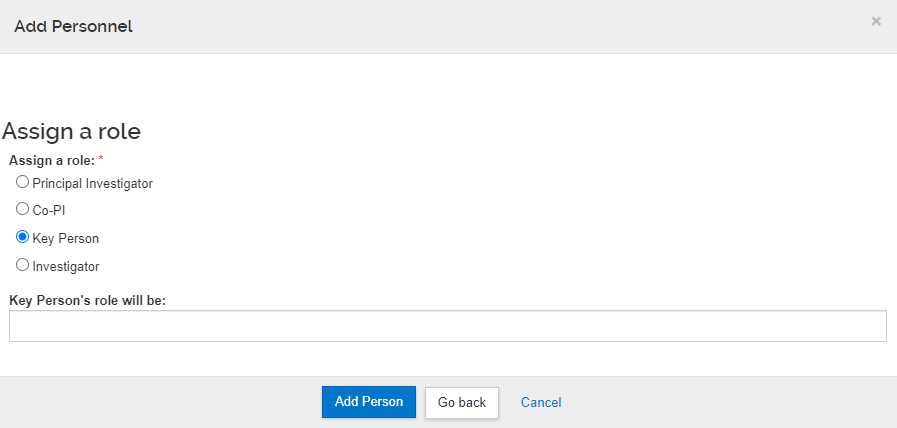
- When Key Person is selected, the Key Person Role field will appear and must be completed.
- Proposal Roles can be reassigned in the Details tab after proposal persons are added.
6. Click the Add Person button to complete this task or click ‘Go back’ to open the previous search results window.
Federal Sponsors or Federal Prime Sponsors Certification Requirement
For more information on the Federal Funding Proposal Review, read through the Research Security & Integrity Compliance website: https://research.uci.edu/rsie/fedprop-review/
When either a federal sponsor or federal prime sponsor is selected, it will now be required that all key personnel must fill out the certification questions prior to the proposal being routed to workflow. Delays in personnel certification could cause delays in proposal submission.
Please refer to the new guidance regarding the KR Personnel Roles, including the new ‘Other Significant Contributor’ role, with details on when each role should be used and their corresponding certification requirements.
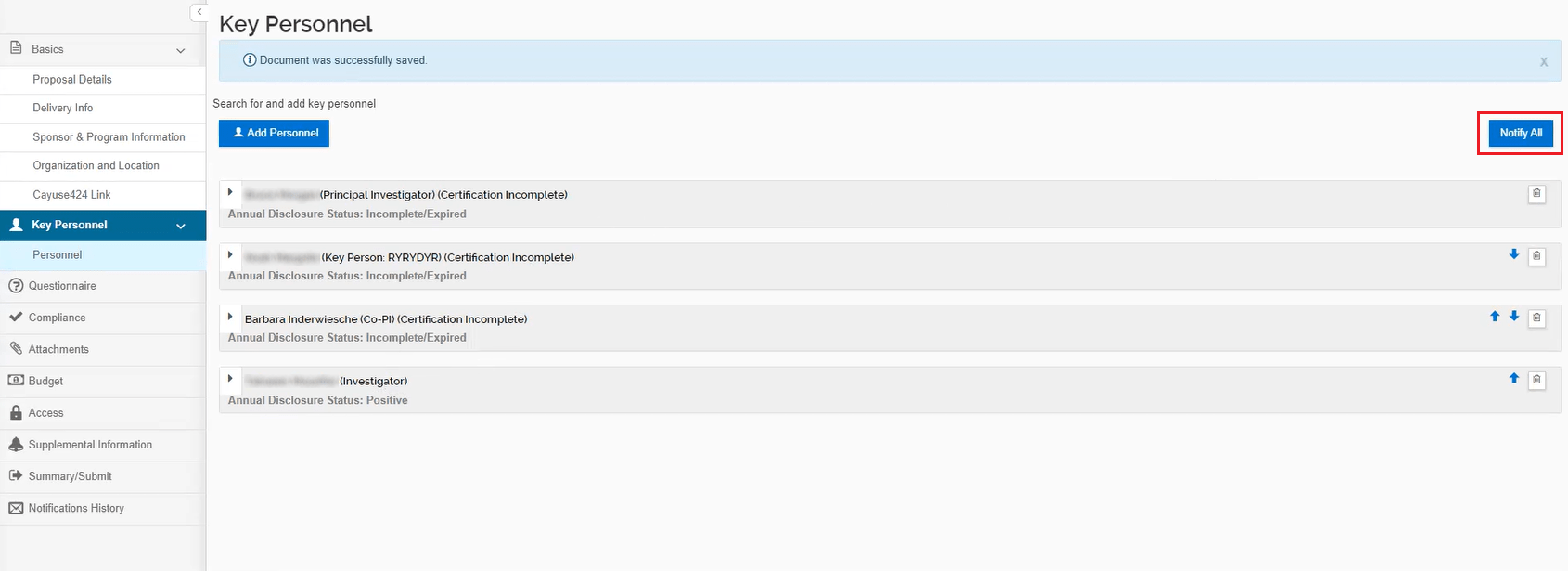
Once all key personnel have been added, select the “Notify All” button to send a specialized notification to the Key Personnel. You can select all personnel to send the notification and you can come back to this page as often as needed to send additional notifications.
Only those assigned to the key personnel role will be required to complete the certification questions. Individuals assigned the Investigator role will not have to answer these questions.
IMPORTANT: Nobody can certify on the key person’s behalf. There are no proxies and no ERA Superuser capabilities. The person that was added on the personnel tab in KR PD, must log in and answer the certification questions.

Each key person must use the link in the notification email that will direct them to the certification questions.
Note: The initiator receives a copy of the notification email as well. This is so that they can also have a link and forward it to the personnel if needed.
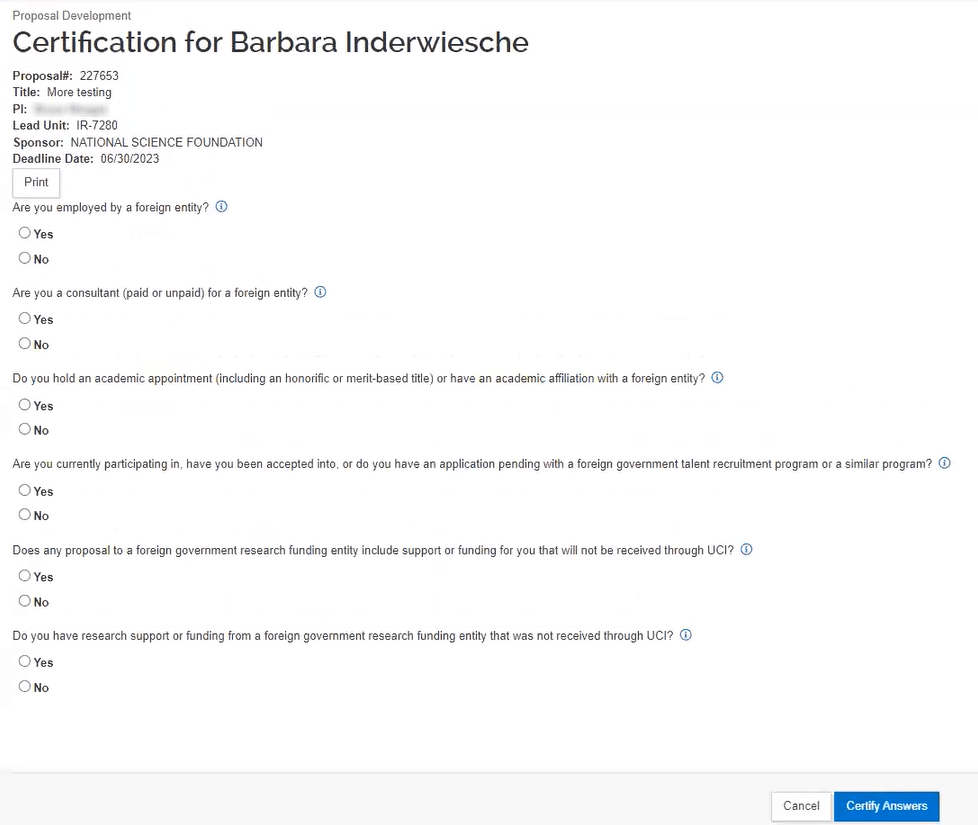
Once the key person follows the link in the notification email, they will log in and be presented with the questions that need to be answered.
It is okay if the key personnel are answering the questions while the proposal development document is still being worked on. Answering the certification questions will not cause any document locks. It is recommended for the Key Personnel to answer the questions as soon as they are added to the proposal so that it is completed and out of the way.
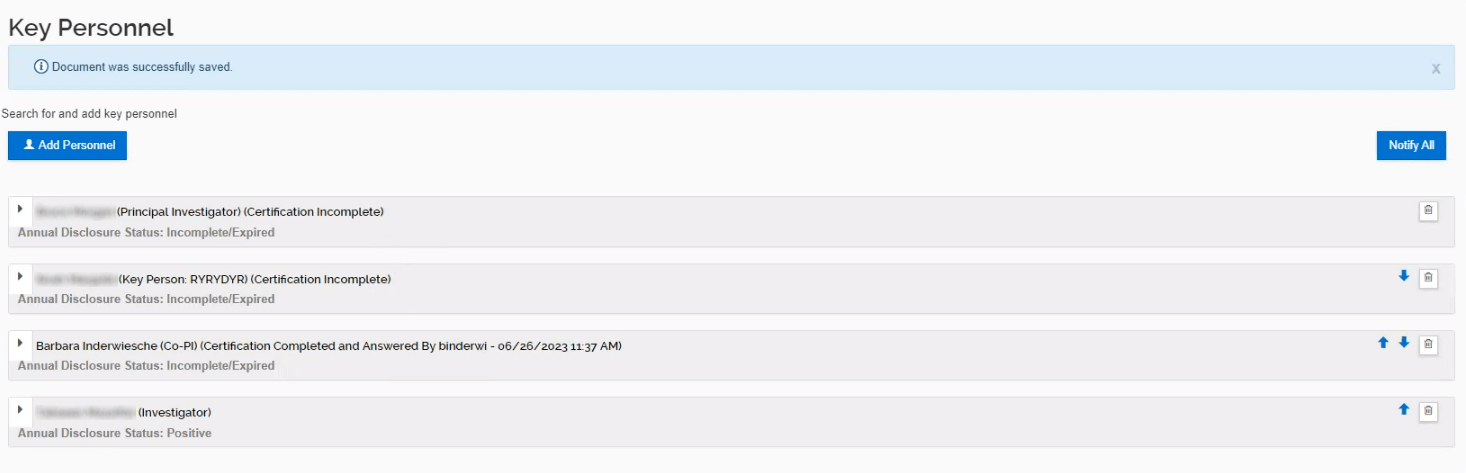
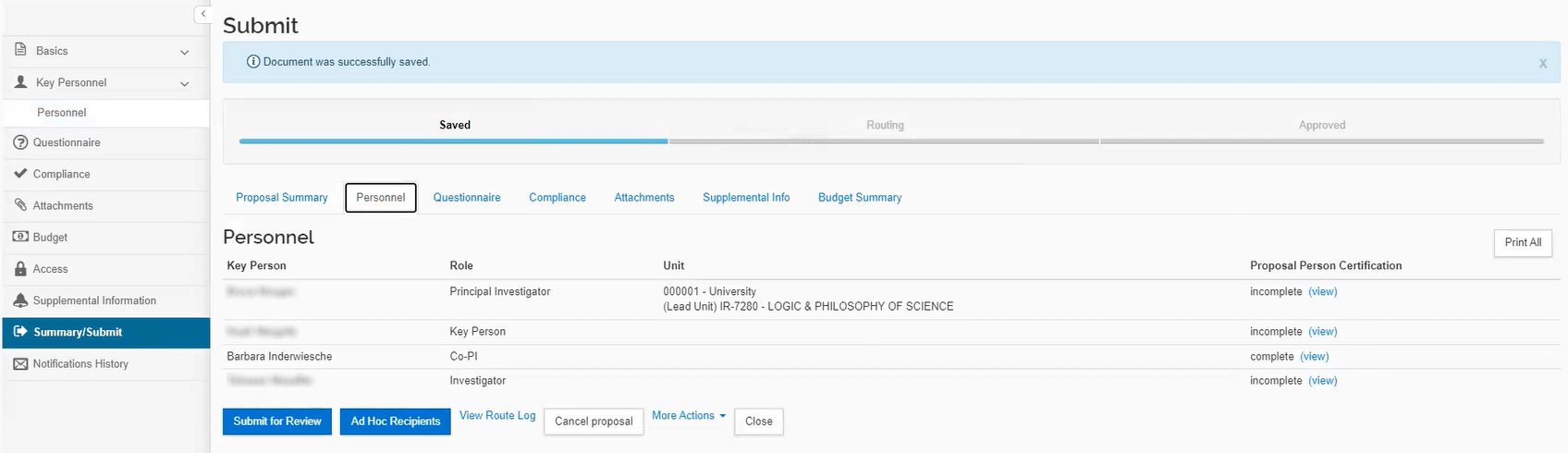

The certification for all Key Personnel must be completed prior to the Initiator submitting the proposal into workflow. If the certification is not completed, the data validations will prevent the proposal from submission into workflow.

Once all Key Personnel have completed their certification, a notification will be sent to the Initiator.
Compliance
The Compliance screen is used to provide relevant research compliance-related information to SPA.
To add Compliance items, click on "Add Compliance Entry" and a pop-up will launch:
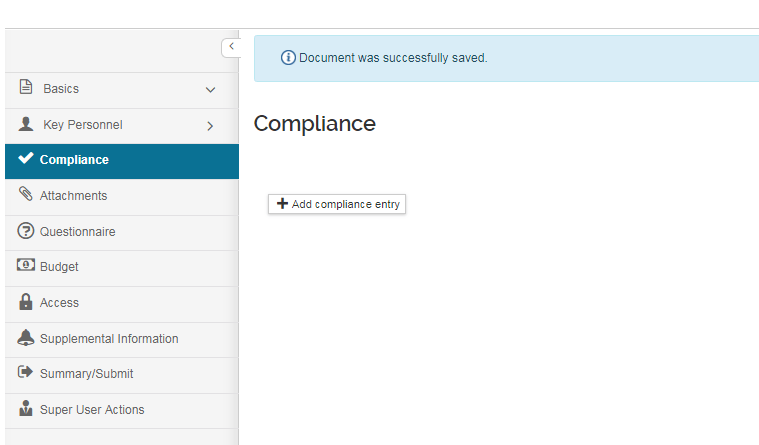
On the "Add Compliance Entry" popup screen, multiple special reviews of the same type can be added if appropriate for the application. Below outlines how to add/remove a Compliance entry and the field options that become available:
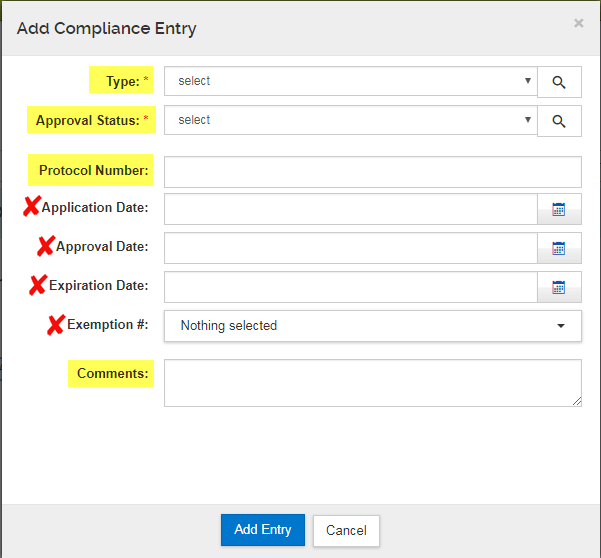
- Type: Select the compliance item you will include. Do not use any of the type denoted for SPA ONLY.
- HSCRO
- IACUC
- IBC
- IRB
- Outgoing Subaward
- Other
- Approval Status: select as applicable.
- Approved
- Pending
- Approval Not Required (do not use - for SPA use only)
- Incomplete (do not use - for SPA use only)
- Protocol Number
- Enter the protocol number if available.
- Use this field to name the Subaward Entity if you selected "Outgoing Subaward" (20 character limit)
- Please see the Subawards section in the Additional Information section below for instructions on how to include subaward information in your proposal document.
- Application Date: Leave blank.
- Approval date: Leave blank.
- Expiration Date: Leave blank.
- Exemption Number: Leave blank.
- Comments: Provide additional comments, if necessary.
Attachments
Proposal
Attach a copy of the proposal file for review. Set the "Status" for the attachment is "Complete."
*Note: If a Proposal Cover Letter or Signed Commitment Form is needed from SPA, please upload a placeholder using Attachment Type: "Proposal Cover Letter" so that SPA can replace it with a signed letter/form upon review. SPA does NOT have the ability to upload additional attachments in the Proposal Attachments section but can replace existing documents.
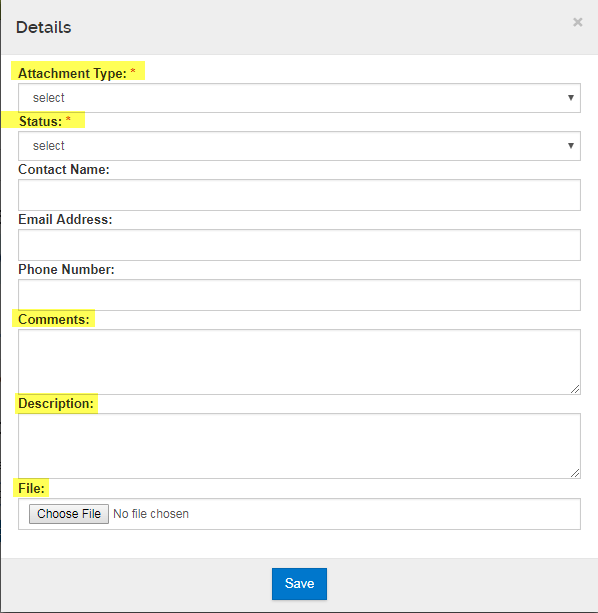

- Attachment Type: Select the attachment type applicable
- Grants.gov Package - DRAFT Scope of Work
- Grants.gov Package - FINAL Scope of Work
- Proposal Package complete per RFA - DRAFT Scope of Work
- Proposal Package complete per RFA - FINAL Scope of Work
- Proposal Cover Letter
- Budget
- MCA/Subrecipient Commitment form
- Other proposal attachment
- Status: Always set as "Complete"
- Contact name: Not required, leave blank.
- Email Address: Not required, leave blank.
- Phone Number: Not required, leave blank.
- Comments: Provide comments, if necessary.
- Description: Provide description of attachment (optional)
Personnel
Not required; Leave blank.
Abstracts
Not required; Leave blank.
Internal
Attach any documents that are not part of the proposal file to be submitted to the sponsor here. This may include internal budgets, COI forms, copy of Sponsor Guidelines, etc.
*Note: upon SPA's institutional review, if the KR proposal is returned to initiator for edits and SPA attaches a Proposal Return Attachment, it will appear here, in the Internal Attachments section.
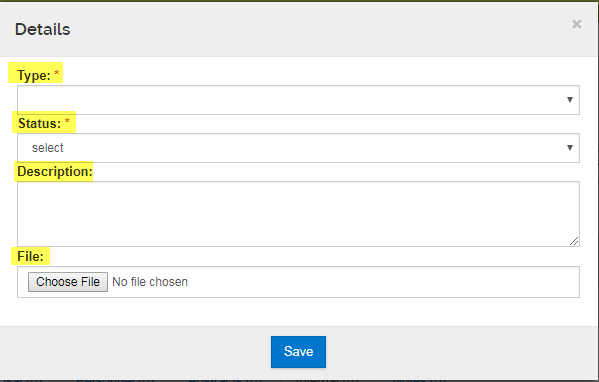
- Type: Select the attachment type applicable
- California 700U
- Indirect Cost Exception Request
- Form 800SR for Non-UCI Investigators
- Form 900SR for Non-UCI Investigators
- Subrecipient Proposal Package
- PI Clinical Trial Questionnaire
- Sponsor Guidelines
- Draft Agreement
- Third Party Cost Share Commitment Letters
- Off-Campus Rate Request
- Clinical Trial Protocol
- VCR Cost Share Commitment Letter
- Other Internal Attachment
- Status: Always set as "Complete"
- Description: Provide description of attachment (optional)
Notes
You may add notes regarding the proposal and or attachments to this section, if necessary.
Please be aware that notes CANNOT be deleted.
Click the "+ Add Note" button to add a note. Enter a note topic and note text.

After the note has been added, it will appear as shown in the screenshot below.

Questionnaire
There are four categories of questions: General Questionnaire, Clinical Trial, Compliance & EH&S (Environmental Heath & Safety). All questionnaires must be completed.
Some questions are required for all proposals, and other questions will become conditionally required if answered "yes".

Budget
Only a Summary Budget is only required. Do not complete a Detailed Budget. Do not select Modular Budget option.
For NIH proposals, you will be presented with the option to select a Modular Budget; do not select Modular Budget, even if you are assembling a Modular Budget in Grants.gov or Cayuse.
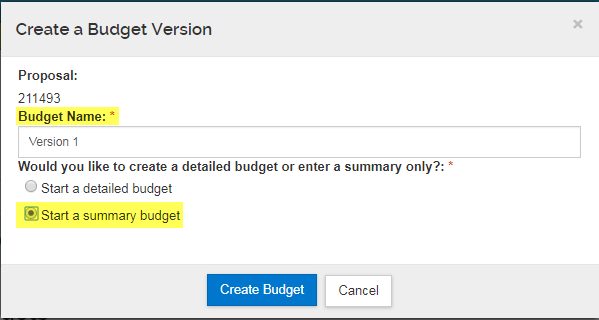
To add a budget, first click "Add Budget":

Then provide a Budget Name, select "Start a Summary Budget" (do not select 'Start a detailed budget') and then Click "Create Budget":
On the Periods & Totals tab, you only need to enter dollar amounts in the Direct Cost and F&A Cost columns.
The F&A Rate and Rate Type are not entered here, but will be entered on the Supplemental Information tab instead.
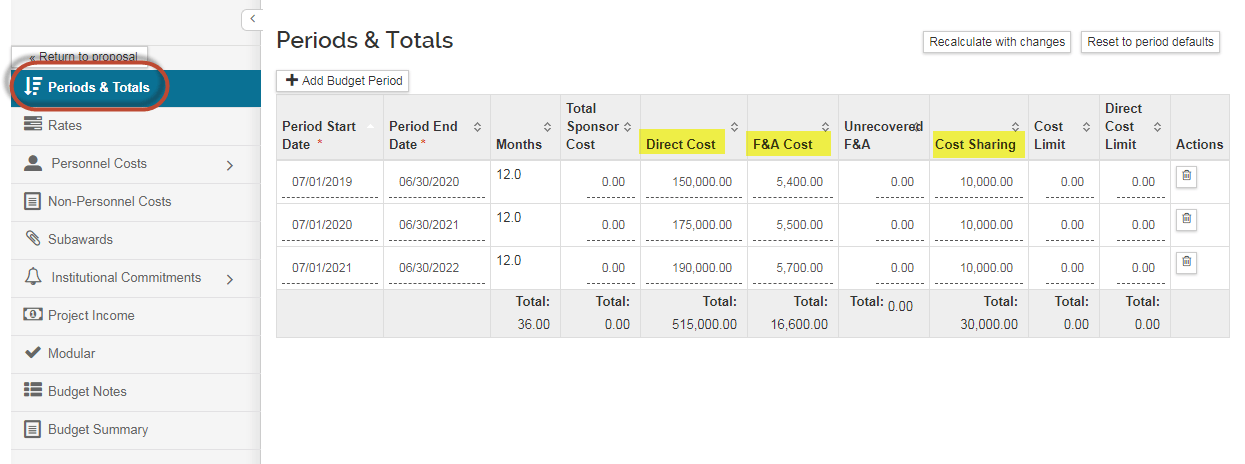
If there is Mandatory Committed Cost Share, include that amount in the Cost Sharing column. If a value is entered here, you will need to complete the Institutional Commitments > Cost Sharing Section:
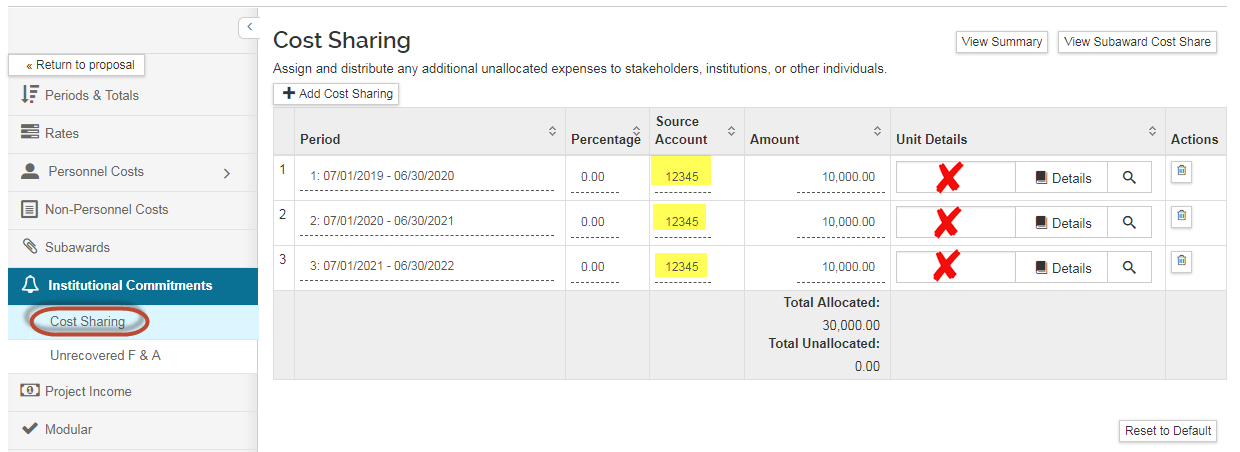
- Period: Based on what is entered on the summary page.
- Percentage: Leave as is.
- Source Account: this field is required in order to save; but we are currently not utilizing the field. Enter "12345" as a placeholder.
- Amount: Based on what is entered on the summary page
- Unit Details: Leave blank.
Once the budget amounts have been entered, Click "Complete Budget":
Next, select the checkbox that indicates the budget is ready to be submitted to the sponsor . Then click "OK."
You can now click "Return to Proposal."
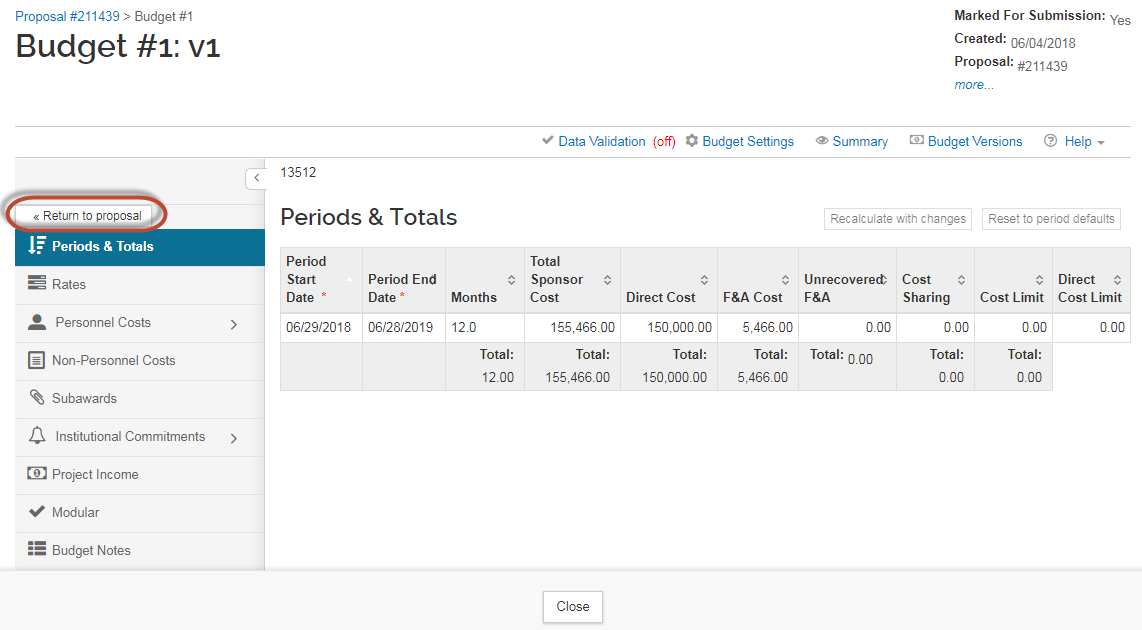
All other sections of the budget (Personnel Costs, Non-Personnel Costs, Subawards...) are NOT required and should be left empty.

Note: If your proposal includes Subawards, do not include the subaward budget attachments here. They should all be included in the Subrecipient Proposal Package to be attached on the Internal Attachments section of the Attachments page. Please see the Subawards section in the Additional Information section below for instructions on how to include subaward information in your proposal document.
Access
You can grant access to this proposal to other users if necessary. However, to obtain global access to proposals for your department, refer to the Logging In section for instructions on obtaining Proposal Creator and/or Admin Contact role access in KSAMS.

Supplemental Information
This section contains additional fields that have to be completed.
- Sponsor Contact Information: in cases where a cover letter must be provided by SPA, enter the Sponsor Contact info so that your SPA officer knows who to address the letter to
- On/Off Campus: Indicate where the majority of the proposed project will take place. This will help in determining whether the correct F&A Rate is being applied.
- F&A Rate: Provide the F&A Rate included in the proposal budget. This field is for numbers only. Do not include the % sign or any alpha characters.
- F&A Rate Type: Provide the F&A Rate Type included in the proposal budget.

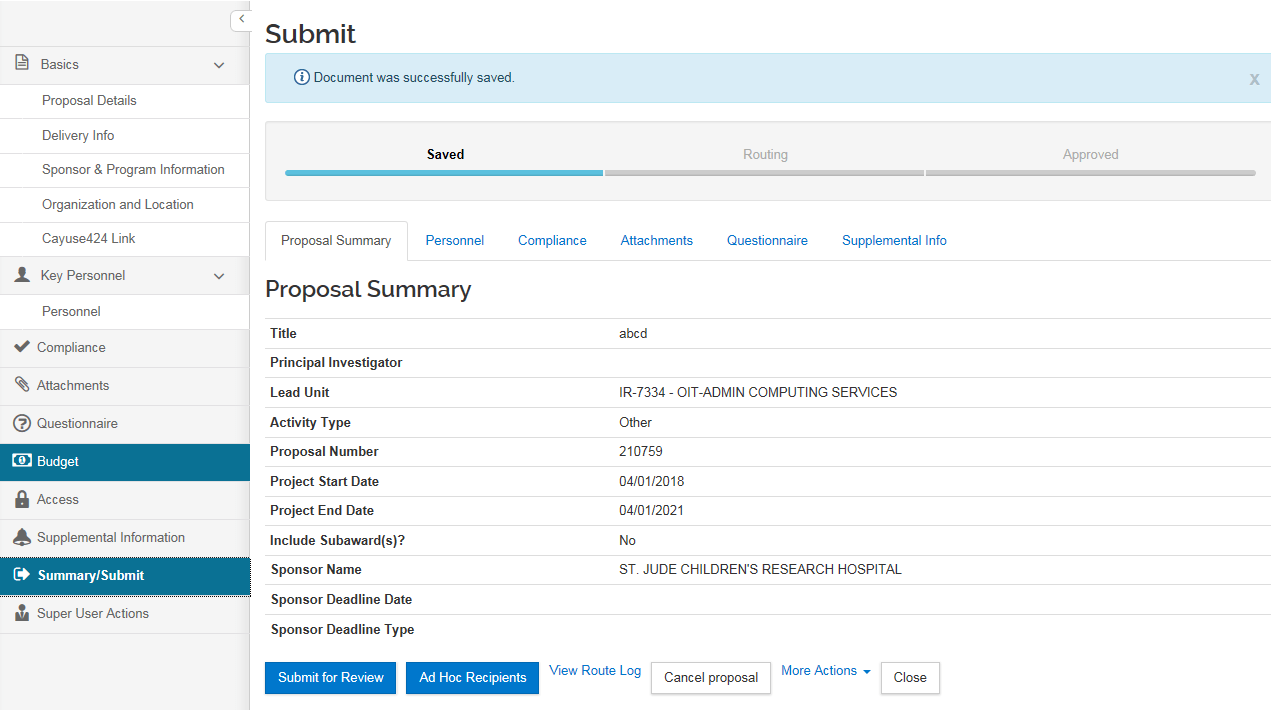
Summary/Submit
The Summary/Submit screen provides a series of tabs with information across the entire proposal so a user can better review the proposal from this single tab. Proposal preparers can review the summary for completion prior to submitting into workflow.
Additional Information
(Proposals with Subawards, Link Feature, Summary/Submit, Approver Actions)
Proposals with Subawards
For proposals that include subawards, follow the instructions below:
- Basics tab > Sponsor & Program Information:
- Check the Subawards checkbox (Yes, this proposal includes subaward(s))
- Attachments tab:
- Attach all Subrecipient package documents to the Internal Attachments page
- Compliance tab:
- Add a compliance entry by selecting "Outgoing Subaward" as the compliance type
- Enter the subaward entity name on the Protocol Number field
- If the subaward site is following UCI's COI policy, add "-COI" to the entity name in the Protocol Number field
- DO NOT enter any Subaward information or attachments on the Budget forms
Link Feature
In the top toolbar, there is a "Link" button that you can click. This is similar to the "Share Proposal" button on the Personnel tab in the old KC system. You can use this button to generate a direct link to this proposal in KR which you can share with your colleagues or PI if they are unable to access or find the proposal in the system.

Summary/Submit Actions
The available action buttons along the bottom change depending on the route status of a proposal (i.e. In Progress, Enroute, Final, etc.). Below outlines the action button options at the various stages. Please note that some of these buttons will only be available to users with the appropriate permissions.
Prior to Submitting for Review:
- Submit for Review: Submits the proposal into workflow for review and approval. All validations will be processed prior to routing. Proposals may be submitted with warnings; click the button in the validation window to “submit with warnings” or close the validation window to address the warnings. Errors, however, must be fixed prior to submission into workflow.
- Ad Hoc Recipients: Allows a user to create custom routing insertions on the proposal - more information on this functionality below.
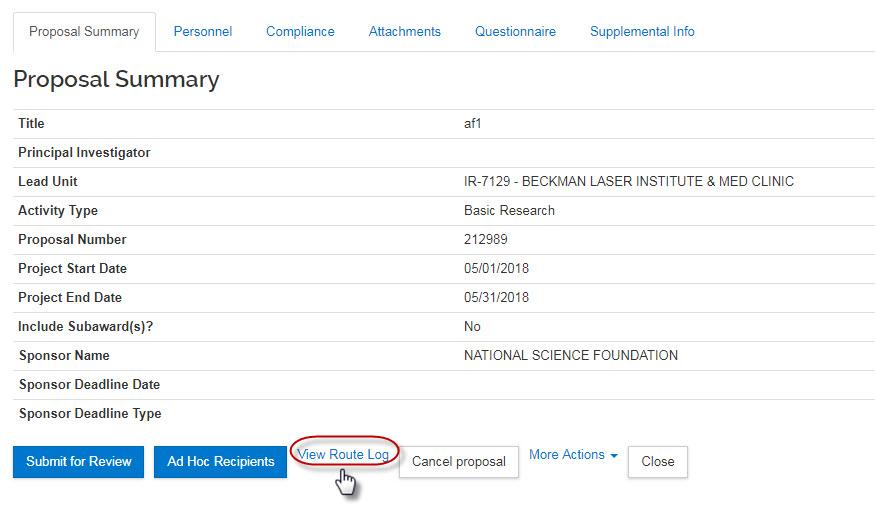
- View Route Log: Users with access to the proposal can click the View Route Log link to view the routing progress, view which approvers have already approved and approvals are still pending.
- Cancel Proposal: Click this button to cancel the proposal document - once canceled the proposal can't be routed or edited but can still be copied.
- More Actions:
- -> Send Notifications: Currently not used.
- -> Reload Proposal: Will reload the proposal from the last save - any changes since will not be saved.
- Close: Click this button to exit the proposal document. Click OK in the confirmation to save any recent changes.
After Submitting for Review:
- Send Adhoc: Will send proposal to the added Ad Hoc Recipients and insert them in the workflow - more information on this functionality below.
- Ad Hoc Recipients: Allows a user to create custom routing insertions on the proposal - more information on this functionality below.
- View Route Log: Users with access to the proposal can click the View Route Log link to view the routing progress, view which approvers have already approved and approvals are still pending.
- Recall: Allows an aggregator to pull back the proposal from workflow and return it to the their action list for edit. When the Recall button is clicked the user must enter a reason for the recall in the confirmation window and then click “ok” to complete the action. Otherwise, click ‘cancel.’
- If any approvals have already been completed, they will need to be obtained again.
- More Actions:
- -> Send Notifications: Currently not used.
- Close: Click this button to exit the proposal document. Click OK in the confirmation to save any recent changes.
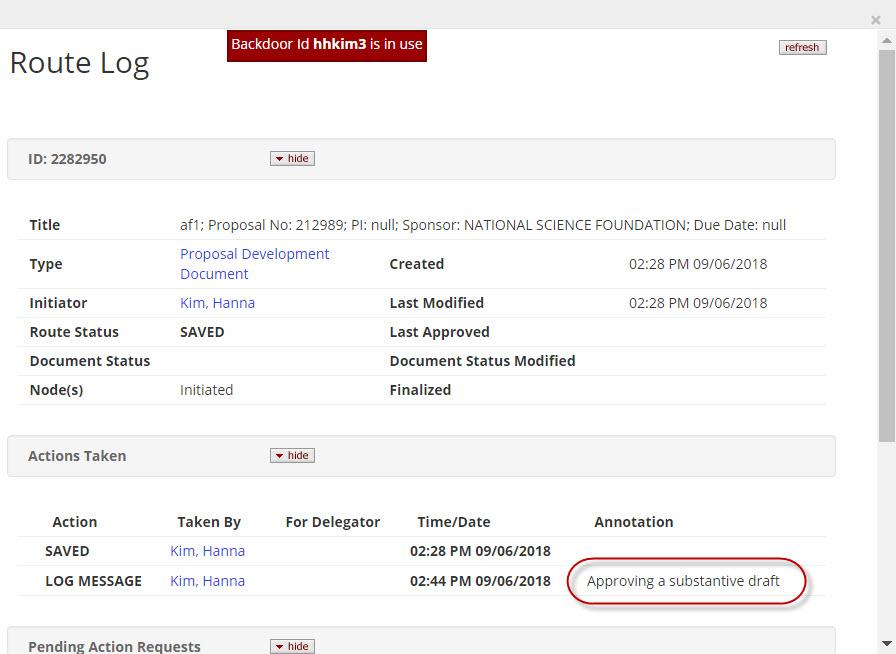
Reviewer/Approver Actions
Approvers will see documents requiring their attention in their Action List (button in top right hand corner). Also, the user should receive an email notification depending on your institutional configurations. Once in a document the approver may click through the tabs in the Submit screen to view the summarized information in the proposal without having to leave this screen, however, they also can navigate to each tab of the proposal if they choose. The following are the approver options when a document is routed:
- Send Adhoc: Will send proposal to the added Ad Hoc Recipients and insert them in the workflow - more information on this functionality below.
- Ad Hoc Recipients: Allows a user to create custom routing insertions on the proposal - more information on this functionality below.
- Approve*: This signifies your approval of the proposal and allows it to continue along the workflow path. You may receive a message asking if you wish to receive future approval requests if you also appear in a future workflow stop - clicking yes will require you to approve again at the future stop, whereas, clicking no will automatically approve on your behalf at the future stop.
- Return: If the proposal needs corrections, you can return the proposal back to the initiator and include comments.
- View Route Log: Users with access to the proposal can click the View Route Log link to view the routing progress, view which approvers have already approved and approvals are still pending.
- More Actions:
- -> Send Notifications: Currently not used.
- Close: Click this button to exit the proposal document. Click OK in the confirmation to save any recent changes.
KR Approval Language updated 05/10/2024
---
*By approving this proposal, I certify, to the best of my knowledge, that:
-
- The information entered in the Kuali Research Proposal Development (KRPD) system regarding this proposal is true, accurate, and complete.
- The information contained in the proposal application is true and accurate, and that I understand that any false, fictitious, or fraudulent statements or claims may subject the University and me, individually, to criminal, civil, or administrative penalties.
- The information contained in the proposal application is complete and includes all information required in accordance with application guidelines and sponsor rules and policies, and that applicable university policies and guidance were followed in preparing the proposal application.
- No UCI employee or appointee who is proposed to conduct the research is currently debarred, proposed for debarment, suspended, declared ineligible, or voluntarily excluded from transactions by any U.S. federal agency or entity.
- If the proposal is to the U.S. federal government and the proposed research includes either human subject research or research using animals covered by either the US Department of Agriculture or U.S. Public Health Service policies, the IRB and/or IACUC protocol application(s) will be identical in principle and congruent with the UCI scope of work contained in the proposal.
- If the proposal is to a U.S. federal agency, no senior/key personnel is participating in or a party to a Malign Foreign Talent Recruitment Program.
I further certify, that I will comply with all applicable sponsor policies and regulations, university policies and guidance (including the UC Standards of Ethical Conduct and UC Contract and Grant Manual), and applicable law related to this sponsor’s funding decision process.
In addition to the above, and if the proposal is funded, I certify and affirm that:
-
- I accept responsibility for the design, ethical conduct, fiscal and administrative management, and reporting of this project, including submission of any required reports.
- I will comply with the award terms and conditions, sponsor policies and regulations, university policies and guidance (including the UC Standards of Ethical Conduct and UC Contract and Grant Manual), and applicable law, and I will inform all other UCI employees, appointees, and students who will conduct the research project that the same is expected of them.
- I and the research team will follow our unit’s/units’ data security management plan(s) if the sponsored research award involves P3 or P4 data.
- I will submit or will have the protocol Lead Researcher(s) submit modifications and/or changes to the IRB and/or IACUC, as necessary and applicable, to assure the protocol(s) remain identical in principle and congruent with UCI’s scope of work contained in the sponsored research award.
- I, and all personnel on the project, have signed either the new Patent Acknowledgement form (applicable to all new employees, appointees, and visitors as of 11/1/2011) or the Patent Acknowledgment form 10/1/1997 and the Amendment thereto.
- Space arrangements are in place to meet the proposed research project’s space requirements.
- The resources described in the proposal will be available to conduct the research project.
- If any UCI employee, appointee, or student involved in conducting the research project becomes debarred or is proposed for debarment, or is suspended, declared ineligible, or voluntarily excluded from transactions by any U.S. federal agency or entity, that I will promptly report this to Sponsored Projects Administration upon becoming aware of this information.
-----
KR Approval Language updated 11/09/2020
All approval stops:
Approval indicates that information in the proposal and KR is complete and accurate to the best of your knowledge. Approval confirms any cost sharing commitments, space arrangements, resources for the project, and that the PI, and all personnel on the project, have signed either the new Patent Acknowledgement form (applicable to all new employees, appointees, and visitors as of 11/1/2011) or the Patent Acknowledgment form 10/1/1997 and the Amendment thereto . The PI, Co-PI, and others involved in the project are not currently debarred, proposed for debarment, suspended, declared ineligible, or voluntarily excluded from transactions by a federal agency. If awarded, the PI, Co-PI, and lead unit agree to be responsible for the fiscal and scientific conduct of the project as required by law, sponsor regulations, award terms, and university policy. If this proposal is being submitted to a Federal Agency and the scope of work includes human subject research, the PI also certifies that the subsequent IRB application submitted for this project will be identical in principle and congruent with the scope of work outlined in the attached proposal application. Further, the PI will submit modifications and/or changes to the IRB, as necessary, to assure the proposal/award and application continues to be congruent and identical in principle.
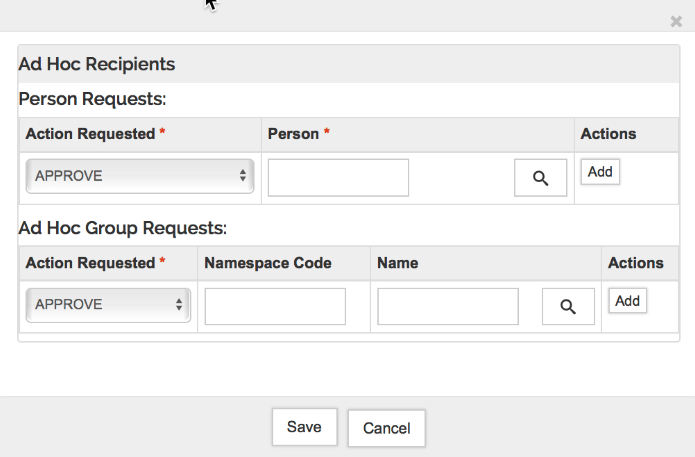
Ad Hoc Recipients
Use this tool to generate custom routing requirements for the proposal. The following types of route stops can be added for an individual person, or to a group:
- FYI - FYIs do not interrupt the normal workflow routing of a document but give a user view access and allows them to signify they've taken action. A document with no pending approval requests but with pending FYI requests is in Final status. The difference between Acknowledgement and FYI is that FYI requests can be cleared directly from the action list without opening the document. FYI requests also have a different effect on the document status than Acknowledgements.
- Acknowledge - Acknowledgements do not interrupt the normal Workflow routing of a document. They do not stop a document from routing on to other individuals or workgroups who need to take approval actions. It allows you to make a user aware of a given proposal and have access to view. However, a document with no pending approval requests but with pending Acknowledge requests is in Processed status.
- Approve - This type of stop will require the user take the approval action prior to it moving forward in workflow. Approval must be done by the individual/group assigned before it can complete or proceed in routing.
- Complete - Only available prior to submitting proposal for review (proposal status is In Progress). This allows a user to ad-hoc route a pending proposal in an editable state to another user to complete the proposal and submit into routing. Be aware, when a user has it in their Action List to complete it's editable but the only option is to submit the proposal for review but it cannot be returned to the initiator.
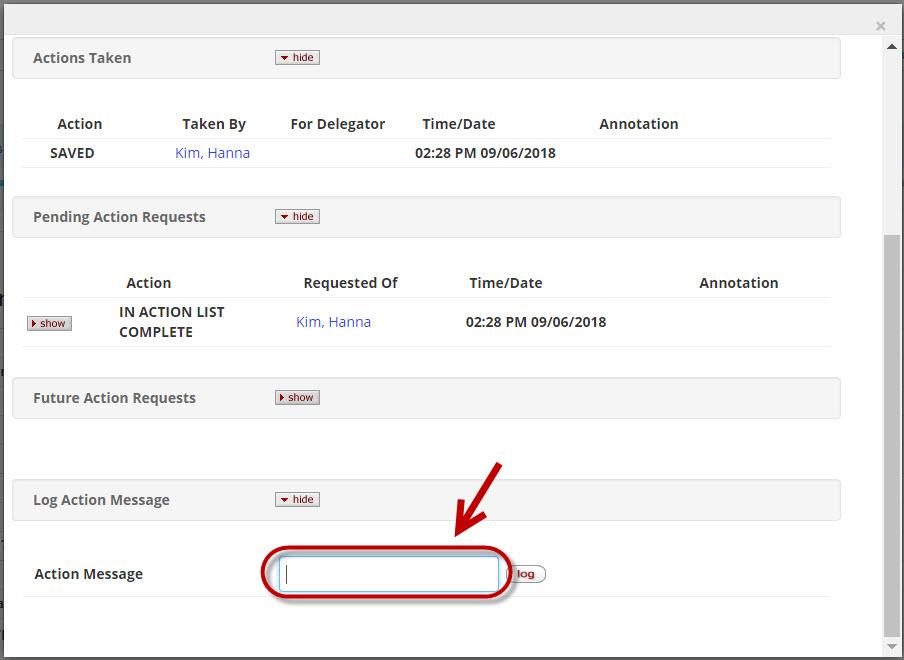
To Create an Ad Hoc Requirement click the Ad Hoc Recipients button to open a new window where you can select the desired Action Request in the Person or Group request section. Use the magnifying glass icon to search for the individual or group you want to ad-hoc route for action. Repeat the steps if more users should be included, otherwise click the Save button to close the window. To actually send the ad-hoc requests and insert them in the route log you'll need to click the 'Send Ad-hoc Requests' button on the bottom of the proposal.
When the notification content is complete and all desired recipients have been added click the 'Send Notifications' button to complete the process. If you wish to send another email you can at any time on a proposal while it's in progress or enroute.



















































